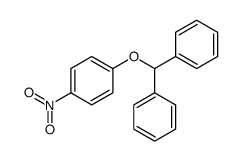
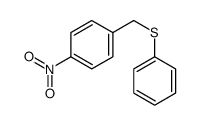
|
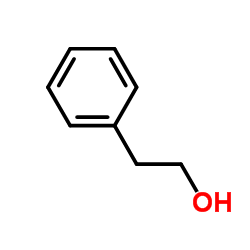
~89% |
|
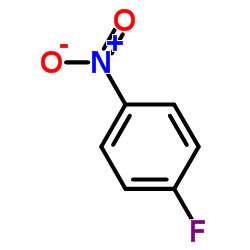
~% |
|
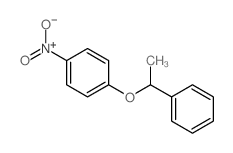
~% |
|
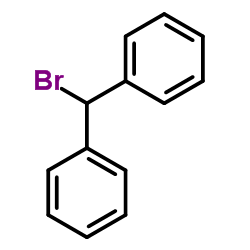
~99% |
|
~62% |
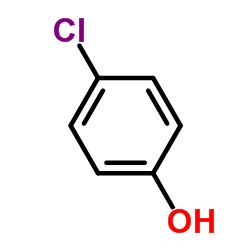
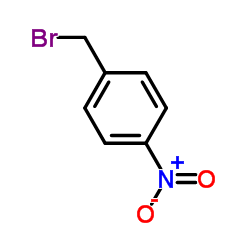
![Benzene, 1-methyl-4-[(4-nitrophenyl)methoxy]结构式](https://image.chemsrc.com/caspic/379/5442-44-4.png)
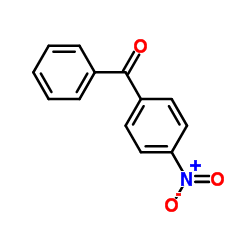
![1-[bromo(phenyl)methyl]-4-nitrobenzene结构式](https://image.chemsrc.com/caspic/159/955-43-1.png)