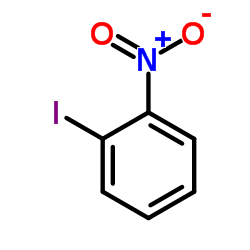
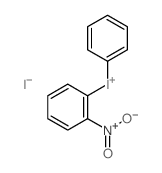
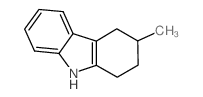
|
~88% |
|
~86% |
|
~% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~87% |

![1,2,3,4-四氢环戊[b]吲哚结构式](https://image.chemsrc.com/caspic/259/2047-91-8.png)