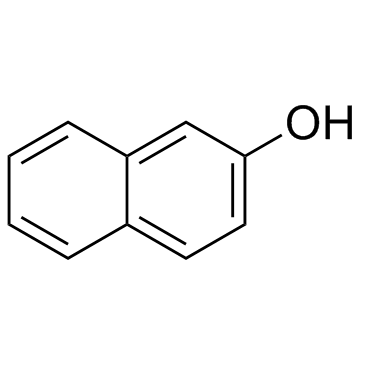
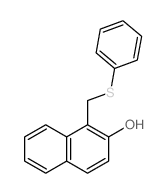
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

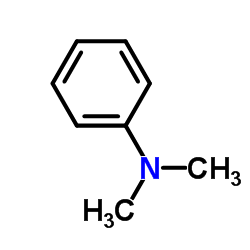
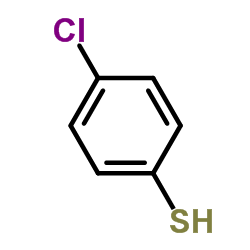
![Benzenamine,4-[[(4-chlorophenyl)thio]methyl]-N,N-dimethyl结构式](https://image.chemsrc.com/caspic/465/959-82-0.png)
![4-[(4-chlorophenyl)sulfanylmethyl]-N-methyl-aniline结构式](https://image.chemsrc.com/caspic/197/7153-31-3.png)
![Benzenamine,3-chloro-N-[[(4-chlorophenyl)thio]methyl]结构式](https://image.chemsrc.com/caspic/313/956-65-0.png)

![4-[(苯基磺酰基)甲基]苯胺结构式](https://image.chemsrc.com/caspic/315/13738-70-0.png)

![Benzenamine,2-chloro-N-[[(4-chlorophenyl)thio]methyl]结构式](https://image.chemsrc.com/caspic/482/956-34-3.png)
![N-[(4-chlorophenyl)sulfanylmethyl]-N-methyl-aniline结构式](https://image.chemsrc.com/caspic/037/956-06-9.png)