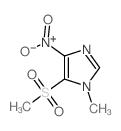
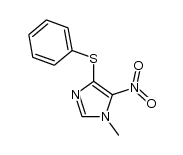
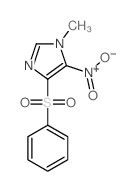
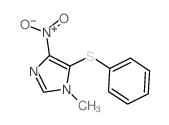
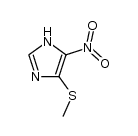
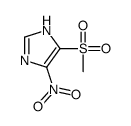
|
~80% |
|
~79% |
|
~% |
|
~75% |
|
~% |
|
~77% |
|
~85% |