Crizotinib (PF-02341066)
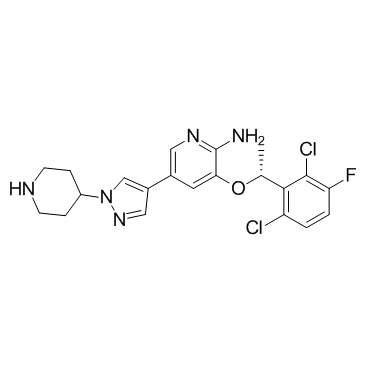
Crizotinib (PF-02341066) structure
|
Common Name | Crizotinib (PF-02341066) | ||
|---|---|---|---|---|
| CAS Number | 877399-52-5 | Molecular Weight | 450.337 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 599.2±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H22Cl2FN5O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 316.2±30.1 °C | |
| Symbol |


GHS07, GHS08, GHS09 |
Signal Word | Warning | |
Use of Crizotinib (PF-02341066)Crizotinib is a potent inhibitor of c-Met and ALK with an IC50 of 11 nM and 24 nM in cell-based assays, respectively. |
| Name | crizotinib |
|---|---|
| Synonym | More Synonyms |
| Description | Crizotinib is a potent inhibitor of c-Met and ALK with an IC50 of 11 nM and 24 nM in cell-based assays, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 11 nM (c-Met), 24 nM (ALK) |
| In Vitro | PF-2341066 displays similar potency against c-Met phosphorylation in mIMCD3 mouse or MDCK canine epithelial cells with IC50 of 5 nM and 20 nM, respectivly. PF-2341066 shows improved or similar activity against NIH3T3 cells engineered to express c-Met ATP-binding site mutants V1092I or H1094R or the P-loop mutant M1250T with IC50 of 19 nM, 2 nM and 15 nM, respectively, compared with NIH3T3 cells expressing wild-type receptor with IC50 of 13 nM. In contrast, a marked shift in potency of PF-2341066 is observed against cells engineered to express c-Met activation loop mutants Y1230C and Y1235D with IC50 of 127 nM and 92 nM, respectively, compared with wild-type receptor. PF-2341066 also potently prevents the phosphorylation of c-Met in NCI-H69 and HOP92 cells, with IC50 of 13 nM and 16 nM, respectively, which express the endogenous c-Met variants R988C and T1010I, respectively[1]. PF-2341066 also potently inhibits NPM-ALK phosphorylation in Karpas299 or SU-DHL-1 ALCL cells with an IC50 of 24 nM. PF-2341066 potently prevents cell proliferation, which is associated with G(1)-S-phase cell cycle arrest and induction of apoptosis in ALK-positive ALCL cells with IC50 of 30 nM, but not ALK-negative lymphoma cells[2]. Besides, PF-2341066 prevents osteosarcoma behavior associated with primary tumor growth (i.e., proliferation and survival) as well as metastasis[3]. |
| In Vivo | PF-2341066 reveals the ability to cause marked regression of large established tumors (> 600 mm3) in both the 50 mg/kg/day and 75 mg/kg/day treatment cohorts, with a 60% decrease in mean tumor volume over the 43-day administration schedule in the GTL-16 model. In an another study, PF-2341066 displays the ability to completely inhibits GTL-16 tumor growth for >3 months, with only 1 of 12 mice exhibiting a significant increase in tumor growth over the 3-month treatment schedule at 50 mg/kg/day. A significant dose-dependent reduction of CD31-positive endothelial cells is observed at 12.5 mg/kg/day, 25 mg/kg/day, and 50 mg/kg/day in GTL-16 tumors, indicating that inhibition of MVD shows a dose-dependent correlation to antitumor efficacy. PF-2341066 displays a significant dose-dependent reduction of human VEGFA and IL-8 plasma levels in both the GTL-16 and U87MG models. Marked inhibition of phosphorylated c-Met, Akt, Erk, PLCλ1, and STAT5 levels is observed in GTL-16 tumors following p.o. administration of PF-2341066[1]. PF-2341066 prevents osteosarcoma behavior associated with primary tumor growth as well as metastasis. In nude mice treated with PF-2341066 via oral gavage, the growth and associated osteolysis and extracortical bone matrix formation of osteosarcoma xenografts are prevented by PF-2341066[3]. Treatment of c-MET-amplified GTL-16 xenografts with 50 mg/kg PF-2341066 elicits tumor regression that is associated with a slow reduction in 18F-FDG uptake and decreases expression of the glucose transporter 1, GLUT-1[4]. |
| Kinase Assay | Cells are seeded in 96-well plates in media supplemented with 10% fetal bovine serum (FBS) and transferred to serum-free media [with 0.04% bovine serum albumin (BSA)] after 24 h. In experiments investigating ligand-dependent RTK phosphorylation, corresponding growth factors are added for up to 20 min. After incubation of cells with PF-2341066 for 1 h and/or appropriate ligands for the designated times, cells are washed once with HBSS supplemented with 1 mM Na3VO4, and protein lysates are generated from cells. Subsequently, phosphorylation of selected protein kinases is assessed by a sandwich ELISA method using specific capture antibodies used to coat 96-well plates and a detection antibody specific for phosphorylated tyrosine residues. Antibody-coated plates are (a) incubated in the presence of protein lysates at 4°C overnight; (b) washed seven times in 1% Tween 20 in PBS; (c) incubated in a horseradish peroxidase-conjugated anti-total-phosphotyrosine (PY-20) antibody (1:500) for 30 min; (d) washed seven times again; (e) incubated in 3,3′,5,5′-tetramethyl benzidine peroxidase substrate to initiate a colorimetric reaction that is stopped by adding 0.09 N H2SO4; and (f) measured for absorbance in 450 nm using a spectrophotometer. |
| Cell Assay | Tumor cells are seeded in 96-well plates at low density in media supplemented with 10% FBS (growth media) and transferred to serum-free media (0% FBS and 0.04% BSA) after 24 h. Appropriate controls or designated concentrations of PF-2341066 are added to each well, and cells are incubated for 24 to 72 h. Human umbilical vascular endothelial cells (HUVEC) are seeded in 96-well plates in EGM2 media for 5 to 6 h at > 20,000 cells per well and transferred to serum-free media overnight. The following day, appropriate controls or designated concentrations of PF-2341066 are added to each well, and after 1 h incubation, HGF is added to designated wells at 100 ng/mL. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay is done to determine the relative tumor cell or HUVEC numbers. |
| Animal Admin | Athymic mice bearing xenografts (300-800 mm3) are given PF-2341066 in water by oral gavage at designated dose levels. At designated times following PF-2341066 administration, mice are humanely euthanized, and tumors are resected. Tumors are snap frozen and pulverized using a liquid nitrogen-cooled cryomortar and pestle, protein lysates are generated, and protein concentrations are determined using a BSA assay. The level of total and phosphorylated protein is determined using a capture ELISA or immunoprecipitation-immunoblotting method. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 599.2±50.0 °C at 760 mmHg |
| Molecular Formula | C21H22Cl2FN5O |
| Molecular Weight | 450.337 |
| Flash Point | 316.2±30.1 °C |
| Exact Mass | 449.118530 |
| PSA | 77.99000 |
| LogP | 4.73 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.673 |
| Storage condition | -20°C |
| Symbol |



GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319-H341-H400 |
| Precautionary Statements | P273-P280-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Safety Phrases | 24/25 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Metabolism, excretion and pharmacokinetics of [14C]crizotinib following oral administration to healthy subjects.
Xenobiotica 45(1) , 45-59, (2014) 1. Crizotinib (XALKORI®), an oral inhibitor of anaplastic lymphoma kinase (ALK) and mesenchymal-epithelial transition factor kinase (c-Met), is currently approved for the treatment of patients with no... |
|
|
Evidence Suggesting That Discontinuous Dosing of ALK Kinase Inhibitors May Prolong Control of ALK+ Tumors.
Cancer Res. 75 , 2916-27, (2015) The anaplastic lymphoma kinase (ALK) is chromosomally rearranged in a subset of certain cancers, including 2% to 7% of non-small cell lung cancers (NSCLC) and ∼70% of anaplastic large cell lymphomas (... |
|
|
Understanding the Interplay between Expression, Mutation and Activity of ALK Receptor in Rhabdomyosarcoma Cells for Clinical Application of Small-Molecule Inhibitors.
PLoS ONE 10 , e0132330, (2015) Receptor tyrosine kinases (RTKs) have a central role in cancer initiation and progression, since changes in their expression and activity potentially results in cell transformation. This concept is es... |
| Crizotinib |
| Xalkori |
| 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-ylpyrazol-4-yl)pyridin-2-amine |
| 3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-2-pyridinamine |
| (R)-3-(1-(2,6-Dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine |
| 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine |
| 2-Pyridinamine, 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]- |
| (R)-Crizotinib |
| PF-2341066 |
| PF2341066 |

