5-benzylacyclouridine
Modify Date: 2024-01-09 17:30:19
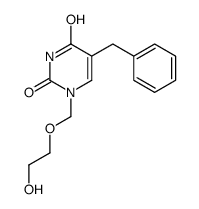
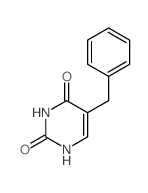
5-benzylacyclouridine structure
|
Common Name | 5-benzylacyclouridine | ||
|---|---|---|---|---|
| CAS Number | 82857-69-0 | Molecular Weight | 276.28800 | |
| Density | 1.29g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C14H16N2O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of 5-benzylacyclouridineBenzylacyclouridine (BAU) is a potent and specific inhibitor of uridine phosphorylase, the first enzyme in the catabolism of uridine. Benzylacyclouridine can modulate the cytotoxic side effects of 5-fluorouracil (5-FU) and its derivatives[1][2][3]. |
| Name | 5-benzyl-1-(2-hydroxyethoxymethyl)pyrimidine-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Benzylacyclouridine (BAU) is a potent and specific inhibitor of uridine phosphorylase, the first enzyme in the catabolism of uridine. Benzylacyclouridine can modulate the cytotoxic side effects of 5-fluorouracil (5-FU) and its derivatives[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
uridine phosphorylase[1] |
| In Vitro | Benzylacyclouridine increases 5-FU-induced cytotoxicity in a number of human cancer cell lines[1]. Benzylacyclouridine (20-100 μM) reduces the rapid clearance of trace amounts of either [14C]uridine or hyperphysiologic concentrations of non-labeled uridine by the isolated rat liver perfused with an artificial oxygen carrier[2]. |
| In Vivo | Benzylacyclouridine (p.o. or i.v.) arrests the rapid degradation of a tracer dose of uridine into uracil in dogs and pigs[1]. Benzylacyclouridine exhibits the t1/2 of 1.8-3.6 h in dogs, with bioavailability levels of 85% (30 mg/kg) and 42.5% (120 mg/kg)[1]. Benzylacyclouridine (120 mg/kg) exhibits the t1/2 of 1.6-2.3 h, with a bioavailability of 40% in pigs[1]. |
| References |
| Density | 1.29g/cm3 |
|---|---|
| Molecular Formula | C14H16N2O4 |
| Molecular Weight | 276.28800 |
| Exact Mass | 276.11100 |
| PSA | 84.58000 |
| LogP | 0.50610 |
| Index of Refraction | 1.583 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933599090 |
|
~% 
5-benzylacyclou... CAS#:82857-69-0 |
| Literature: Orr, G. Faye; Musso, David L.; Kelley, James L.; Joyner, Suzanne S.; Davis, Stephen T.; Baccanari, David P. Journal of Medicinal Chemistry, 1997 , vol. 40, # 8 p. 1179 - 1185 |
|
~70% 
5-benzylacyclou... CAS#:82857-69-0 |
| Literature: Lin, Tai-Shun; Liu, Mao-Chin Synthetic Communications, 1988 , vol. 18, # 9 p. 931 - 936 |
|
~% 
5-benzylacyclou... CAS#:82857-69-0 |
| Literature: Lin, Tai-Shun; Liu, Mao-Chin Synthetic Communications, 1988 , vol. 18, # 9 p. 931 - 936 |
|
~% 
5-benzylacyclou... CAS#:82857-69-0 |
| Literature: Orr; Musso; Boswell; Kelley; Joyner; Davis; Baccanari Journal of Medicinal Chemistry, 1995 , vol. 38, # 19 p. 3850 - 3856 |
|
~% 
5-benzylacyclou... CAS#:82857-69-0 |
| Literature: Orr; Musso; Boswell; Kelley; Joyner; Davis; Baccanari Journal of Medicinal Chemistry, 1995 , vol. 38, # 19 p. 3850 - 3856 |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 2,4(1H,3H)-Pyrimidinedione,1-((2-hydroxyethoxy)methyl)-5-(phenylmethyl) |
| Benzylacyclouridine |
| 1-((2-HYDROXYETHOXY)METHYL)-5-BENZYLPYRIMIDINE-2,4(1H,3H)-DIONE |
| 5-Benzyl-1-(2'-hydroxyethoxymethyl)uracil |
| 5-Bacu |
| BAU |
| 5-Benzylacyclouridine |