1-Bromo-2-methyl-3-nitrobenzene
Modify Date: 2024-01-05 18:45:45
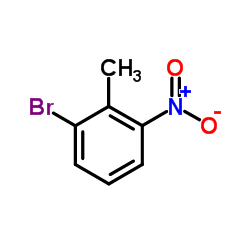
1-Bromo-2-methyl-3-nitrobenzene structure
|
Common Name | 1-Bromo-2-methyl-3-nitrobenzene | ||
|---|---|---|---|---|
| CAS Number | 55289-35-5 | Molecular Weight | 216.03 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 259.6±20.0 °C at 760 mmHg | |
| Molecular Formula | C7H6BrNO2 | Melting Point | 38-40 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 110.8±21.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 1-Bromo-2-methyl-3-nitrobenzene1-Bromo-2-methyl-3-nitrobenzene is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 2-Bromo-6-nitrotoluene |
|---|---|
| Synonym | More Synonyms |
| Description | 1-Bromo-2-methyl-3-nitrobenzene is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 259.6±20.0 °C at 760 mmHg |
| Melting Point | 38-40 °C(lit.) |
| Molecular Formula | C7H6BrNO2 |
| Molecular Weight | 216.03 |
| Flash Point | 110.8±21.8 °C |
| Exact Mass | 214.958176 |
| PSA | 45.82000 |
| LogP | 2.98 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.593 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | UN 2810 6.1/PG 1 |
| WGK Germany | 3 |
| HS Code | 2904909090 |
|
~92% 
1-Bromo-2-methy... CAS#:55289-35-5 |
| Literature: Nicolaou; Snyder, Scott A.; Huang, Xianhai; Simonsen, Klaus B.; Koumbis, Alexandros E.; Bigot, Antony Journal of the American Chemical Society, 2004 , vol. 126, # 32 p. 10162 - 10173 |
|
~% 
1-Bromo-2-methy... CAS#:55289-35-5 |
| Literature: Recueil des Travaux Chimiques des Pays-Bas, , vol. 53, p. 1011,1016, 1025 |
|
~27%
Detail
|
| Literature: Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), , p. 1606 - 1616 |
|
~% 
1-Bromo-2-methy... CAS#:55289-35-5 |
| Literature: Canadian Journal of Chemistry, , vol. 40, p. 511 - 517 |
|
~% 
1-Bromo-2-methy... CAS#:55289-35-5 |
| Literature: Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), , p. 1606 - 1616 |
|
~%
Detail
|
| Literature: Recueil des Travaux Chimiques des Pays-Bas, , vol. 54, p. 235,238 Recueil des Travaux Chimiques des Pays-Bas, , vol. 53, p. 1011,1016, 1025 |
|
~%
Detail
|
| Literature: Recueil des Travaux Chimiques des Pays-Bas, , vol. 54, p. 235,238 Recueil des Travaux Chimiques des Pays-Bas, , vol. 53, p. 1011,1016, 1025 |
|
~%
Detail
|
| Literature: Recueil des Travaux Chimiques des Pays-Bas, , vol. 54, p. 235,238 Recueil des Travaux Chimiques des Pays-Bas, , vol. 53, p. 1011,1016, 1025 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2904909090 |
|---|---|
| Summary | HS:2904909090 sulphonated, nitrated or nitrosated derivatives of hydrocarbons, whether or not halogenated VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
First total synthesis of the neuronal cell protecting carbazole alkaloid carbazomadurin A by sequential transition metal-catalyzed reactions.
Chem. Commun. (Camb.) (10) , 1170-1, (2003) The highly oxygenated neuronal cell protecting carbazole alkaloid carbazomadurin A was synthesized in nine steps and 11% overall yield from isovanillic acid. |
|
|
Palladium-catalyzed reactions in the synthesis of 3-and 4-substituted indoles. 2. Total synthesis of the N-acetyl methyl ester of (±)-clavicipitic acids. Harrington PJ, et al.
J. Am. Chem. Soc. 109(14) , 4335-38, (1987)
|
| 2-Bromo-6-nitrotoluene,2-nitro-6-bromotoluene |
| EINECS 259-566-4 |
| MFCD00009792 |
| 1-Bromo-2-methyl-3-nitrobenzene |
| 2-Bromo-6-nitrotoluene |
| Benzene, 1-bromo-2-methyl-3-nitro- |

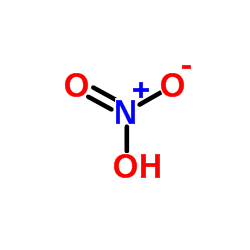
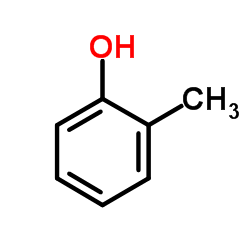
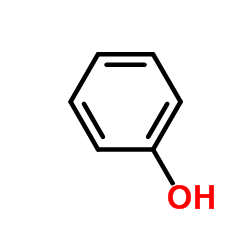
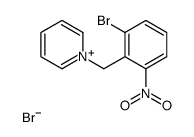 CAS#:109019-72-9
CAS#:109019-72-9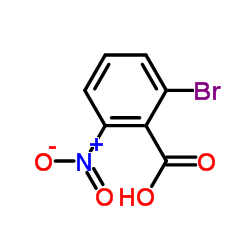 CAS#:38876-67-4
CAS#:38876-67-4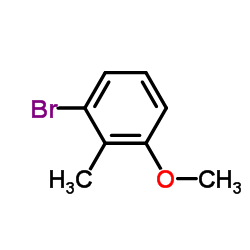 CAS#:31804-36-1
CAS#:31804-36-1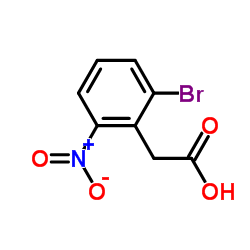 CAS#:37777-74-5
CAS#:37777-74-5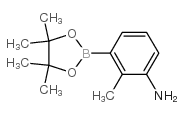 CAS#:882678-96-8
CAS#:882678-96-8 CAS#:98600-34-1
CAS#:98600-34-1 CAS#:7766-23-6
CAS#:7766-23-6 CAS#:20776-48-1
CAS#:20776-48-1 CAS#:77603-45-3
CAS#:77603-45-3 CAS#:885521-72-2
CAS#:885521-72-2
