Lawsone methyl ether
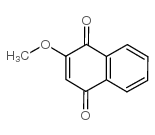
Lawsone methyl ether structure
|
Common Name | Lawsone methyl ether | ||
|---|---|---|---|---|
| CAS Number | 2348-82-5 | Molecular Weight | 188.17900 | |
| Density | 1.28g/cm3 | Boiling Point | 339.8ºC at 760mmHg | |
| Molecular Formula | C11H8O3 | Melting Point | 184-187ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 152.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Lawsone methyl etherLawsone methyl ether (2-Methoxy-1,4-naphthoquinone), isolated from Impatiens balsamina L. and Swertia calycina, exhibits potent antifungal and antibacterial activities[1]. |
| Name | 2-methoxy-1,4-naphthoquinone |
|---|---|
| Synonym | More Synonyms |
| Description | Lawsone methyl ether (2-Methoxy-1,4-naphthoquinone), isolated from Impatiens balsamina L. and Swertia calycina, exhibits potent antifungal and antibacterial activities[1]. |
|---|---|
| Related Catalog | |
| In Vitro | The value of both minimal inhibitory concentration and minimal fungicidal concentration of Lawsone methyl ether (2-Methoxy-1,4-naphthoquinone) against Candida was 1.25 lg⁄ml[1]. |
| References |
| Density | 1.28g/cm3 |
|---|---|
| Boiling Point | 339.8ºC at 760mmHg |
| Melting Point | 184-187ºC(lit.) |
| Molecular Formula | C11H8O3 |
| Molecular Weight | 188.17900 |
| Flash Point | 152.7ºC |
| Exact Mass | 188.04700 |
| PSA | 43.37000 |
| LogP | 1.59590 |
| Vapour Pressure | 8.98E-05mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26;S36 |
| RIDADR | NONH for all modes of transport |
| RTECS | QL8960000 |
| HS Code | 2914690090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914690090 |
|---|---|
| Summary | 2914690090 other quinones。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549 lung adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK signaling pathways.
Life Sci. 135 , 158-64, (2015) The compound 2-methoxy-1,4-naphthoquinone (MNQ) was previously shown to be cytotoxic against several cancer cell lines, but its mode of action is poorly understood. In this study, we aimed to explore ... |
|
|
Anti-gastric adenocarcinoma activity of 2-Methoxy-1,4-naphthoquinone, an anti-Helicobacter pylori compound from Impatiens balsamina L.
Fitoterapia 83(8) , 1336-44, (2012) 2-Methoxy-1,4-naphthoquinone (MeONQ) from Impatiens balsamina L. exhibited strong anti-H. pylori activity in our previous study. In this study, we investigated the cytotoxicity of MeONQ against gastri... |
|
|
Effects of the compounds 2-methoxynaphthoquinone, 2-propoxynaphthoquinone, and 2-isopropoxynaphthoquinone on ecdysone 20-monooxygenase activity.
Arch. Insect Biochem. Physiol. 66(1) , 45-52, (2007) The effects of the natural compound 2-methoxy-1,4-naphthoquinone, isolated from the leaves of Impatiens glandulifera and the synthetic compounds 2-propoxy-1,4-naphthoquinone and 2-isopropoxy-1,4-napht... |
| 2-Methoxy-p-naphthoquinone |
| 1,4-Naphthalenedione,2-methoxy |
| 2-methoxy-1,4-naphthalenequinone |
| 2-methoxynaphthalene-1,4-dione |
| 2-Methoxy-[1,4]naphthoquinone |
| 2-methoxyl-1,4-naphthoquinone |
| 2-Methoxynaphthoquinone |
| 1,4-NAPHTHOQUINONE,2-METHOXY |
| MFCD00019539 |
| 2-Methoxy-1,4-naphthalenedione |
| Methoxy-1,4-naphthochinin |
 CAS#:1010-60-2
CAS#:1010-60-2 CAS#:93-04-9
CAS#:93-04-9 CAS#:57404-85-0
CAS#:57404-85-0 CAS#:1515-76-0
CAS#:1515-76-0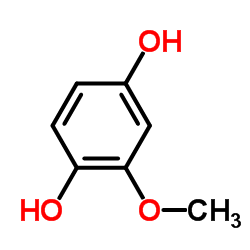 CAS#:824-46-4
CAS#:824-46-4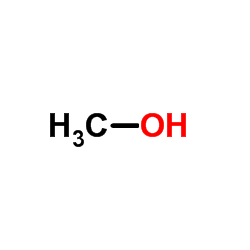 CAS#:67-56-1
CAS#:67-56-1 CAS#:2065-37-4
CAS#:2065-37-4 CAS#:70477-38-2
CAS#:70477-38-2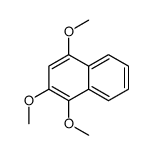 CAS#:60683-53-6
CAS#:60683-53-6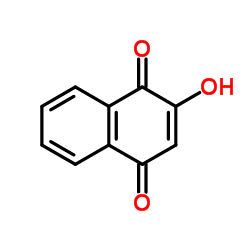 CAS#:83-72-7
CAS#:83-72-7 CAS#:104202-35-9
CAS#:104202-35-9 CAS#:33440-64-1
CAS#:33440-64-1 CAS#:14422-78-7
CAS#:14422-78-7 CAS#:26037-61-6
CAS#:26037-61-6 CAS#:84-79-7
CAS#:84-79-7 CAS#:3408-13-7
CAS#:3408-13-7![1,4-Naphthalenedione,2-[(4-chlorophenyl)amino]- structure](https://www.chemsrc.com/caspic/073/3144-89-6.png) CAS#:3144-89-6
CAS#:3144-89-6 CAS#:17241-45-1
CAS#:17241-45-1 CAS#:24555-42-8
CAS#:24555-42-8![2-[(4-nitrophenyl)amino]naphthalene-1,4-dione structure](https://www.chemsrc.com/caspic/280/75112-65-1.png) CAS#:75112-65-1
CAS#:75112-65-1