101314-97-0
| Name | sodium,(3R,5S)-7-[(1R,2R,6S,8S)-8-(2,2-dimethylbutanoyloxy)-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoate |
|---|---|
| Synonyms |
Sodium (3R,5R)-7-{(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutanoyl)oxy]-2,6-dimethyl-1,2,6,7,8,8a-hexahydro-1-naphthalenyl}-3,5-dihydroxyheptanoate
Simvastatin Hydroxy Acid Sodium Salt simvastatin sodium Si-Methyl-N,N'N''-triphenyl-silantriyltriamin 1-Naphthaleneheptanoic acid, 8-(2,2-dimethyl-1-oxobutoxy)-1,2,6,7,8,8a-hexahydro-β,δ-dihydroxy-2,6-dimethyl-, sodium salt, (βR,δR,1S,2S,6R,8S,8aR)- (1:1) trianilino-methyl-silane simvastatin sodium salt Silanetriamine,1-methyl-N,N',N''-triphenyl Methyl-tris-anilino-silan simivastin sodium salt Simvastatin EP Impurity A Sodium Salt Simvastatin Impurity 1 |
| Description | Simvastatin sodium is a lactone prodrug, can be hydrolysed to active hydroxy-acid by non-specific carboxyesterases or non-enzymatic processes. Simvastatin sodium shows a inhibition of HMG-CoA reductase with a Ki value of 0.12 nM[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Simvastatin sodium shows a inhibition of HMG-CoA reductase in human lymphoblasts and SV40 transformed MRCS fibroblasts[1]. Simvastatin sodium (100 μM) inhibits the Na+/H+ antiport activity leading to a fall of intracellular pH and to a reduced cell proliferation.And the inhibitory effect is prevented by mevalonate but not dolichol or squalene[1]. |
| In Vivo | Simvastatin sodium (oral administration; 8 mg/kg; 18 days) reduces plasma cholesterol levels 33%, simvastatin can be used in combination with ezetimibe to treat dyslipidemia[1]. |
| References |
| Molecular Formula | C25H39NaO6 |
|---|---|
| Molecular Weight | 458.563 |
| Exact Mass | 458.264435 |
| PSA | 106.89000 |
| LogP | 2.77100 |
|
~80% 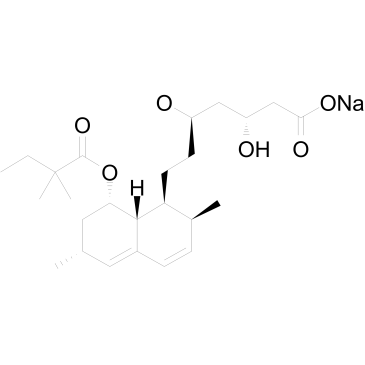
101314-97-0 |
| Literature: Griffin, John; Lanza, Guido; Yu, Jessen Patent: US2005/261354 A1, 2005 ; Location in patent: Page/Page column 53 ; US 20050261354 A1 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |