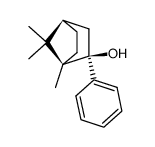
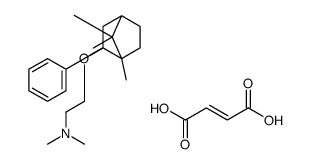
120444-71-5
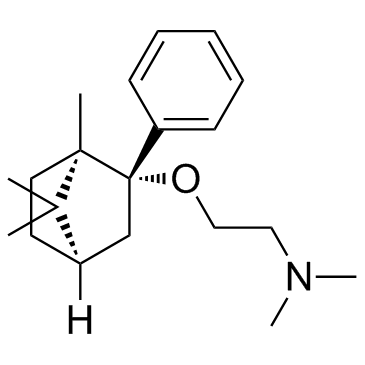
| Name | N,N-dimethyl-2-[[(1R,3S,4R)-4,7,7-trimethyl-3-phenyl-3-bicyclo[2.2.1]heptanyl]oxy]ethanamine |
|---|---|
| Synonyms |
deramciclane,(1R,2S,4R)-isomer
(1R,2S,4R)-(-)-2-[(2'-{N,N-dimethylamino}-ethoxy-)]-2-[phenyl]-1,7,7,-tri-[methyl]-bicyclo[2.2.1]heptane Ethanamine,N,N-dimethyl-2-((1,7,7-trimethyl-2-phenylbicyclo(2.2.1)hept-2-yl)oxy)-,(1R-exo) Deramciclane [14C]-Deramciclane (1R,2S,4R)-(-)-2-phenyl 2-(dimethylaminoethoxy)-1,7,7-trimethyl-bicyclo[2.2.1]heptane Ethanamine,N,N-dimethyl-2-(((1R,2S,4R)-1,7,7-trimethyl-2-phenylbicyclo(2.2.1)hept-2-yl)oxy) (1R,2S,4R)-(-)-2-phenyl-2-(2'-dimethylamino-ethoxy)-1,7,7-trimethyl-bicyclo[2.2.1]heptane Deramciclane [INN] |
| Description | Deramciclane has a high affinity for 5-HT2A and 5-HT2C receptors; it acts as an antagonist at both receptor subtypes and has inverse agonist properties at the 5-HT2C receptors without direct stimulatory agonist. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A receptor, 5-HT2C receptor[1] |
| In Vitro | Deramciclane is a novel anxiolytic agent that binds with high affinity to 5-HT2A/2C receptors. The interactions of Deramciclane with the serotonin 5-HT2C receptor are characterized further using receptor phosphoinositide hydrolysis assays and receptor autoradiography. Deramciclane antagonizes 5-HT2C receptor mediated 5-HT-stimulated phosphoinositide hydrolysis with an IC50 value of 168 nM. Deramciclane also decreases basal phosphoinositide hydrolysis by up to 33% (EC50= 93 nM) in a physiological system in the choroid plexus, suggesting that Deramciclane possesses inverse agonist properties at this receptor[1]. |
| In Vivo | Deramciclane 3 and 10 mg/kg does not change the dopamine levels significantly at any time point versus the basal level whereas 30 mg/kg of Deramciclane significantly increases the levels at 40-100 min and at 160-240 min (P<0.05). Deramciclane is a putative antiserotonergic compound that reduces 5-HT-induced phosphoinositol hydrolysis and a variety of actions caused by serotonergic agonists. The receptor binding profile of Deramciclane is rather similar to that of ritanserin. Deramciclane has a high affinity for 5-HT2A and 5-HT2C receptors; it acts as an antagonist at both receptor subtypes and has inverse agonist properties at the 5-HT2C receptors without direct stimulatory agonist effects. Deramciclane has been shown to have anxiolytic-like activity in several animal tests[2]. |
| Animal Admin | Mice[2] Male Wistar rats are used. Samples for determination of basal levels of dopamine, DOPAC and HVA are collected for 60 min. and after that the drugs (doses refer to the salts) are given intraperitoneally in a volume of 5 mL/kg of body weight. Treatments are Deramciclane fumarate 3 mg/kg (=7.2 µmol/kg), 10 mg/kg (=24 µmol/kg) and 30 mg/kg (=72 µmol/kg); D-amphetamine sulfate 2 mg/kg (=5.4 µmol/kg); Ritanserin 1 mg/kg (=2.1 µmol/kg) and Buspirone hydrochloride 5 mg/kg (=12 µmol/kg). All drugs are suspended in 0.5% carboxymethylcellulose (CMC) dissolved in 0.9% saline. Vehicle control group (n=5) are injected intraperitoneally with 5 mL/kg of 0.5% CMC solution. There are nine rats in each treatment group.The doses of Deramciclane fumarate are considered to produce plasma levels comparable to therapeutic plasma levels in human beings (3 mg/kg) or about three times higher (10 mg/kg) 1-3 hr after the administration of the drug. Behavioural data from earlier Deramciclane studies in rats indicates that Deramciclane has some antidopaminergic activity at high doses (20-40 mg/kg). The highest dose of Deramciclane (30 mg/kg) is selected in this dose range. Fairly high doses of the reference drugs are chosen based on the literature and our own experience to detect the ability of selected drugs to modify extracellular dopamine levels in either the striatum or the nucleus accumbens. After administration of each drug, samples are collected for 240 min. and then divided into two aliquots (35 µL/15 µL). The first aliquot of the samples is stored at 4°C and assayed for dopamine within 24 hr. The other aliquot is frozen and stored at -70°C until assayed for DOPAC and HVA. |
| References |
| Density | 1.01g/cm3 |
|---|---|
| Boiling Point | 375.2ºC at 760mmHg |
| Molecular Formula | C20H31NO |
| Molecular Weight | 301.46600 |
| Flash Point | 110.6ºC |
| Exact Mass | 301.24100 |
| PSA | 12.47000 |
| LogP | 4.30630 |
| Vapour Pressure | 7.93E-06mmHg at 25°C |
| Index of Refraction | 1.541 |
| Storage condition | 2-8℃ |
|
~% 
120444-71-5 |
| Literature: Orion Corporation Patent: US6335469 B1, 2002 ; Location in patent: Example 1 ; |
| Precursor 1 | |
|---|---|
| DownStream 1 | |