58-32-2
| Name | dipyridamole |
|---|---|
| Synonyms |
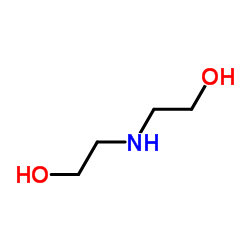
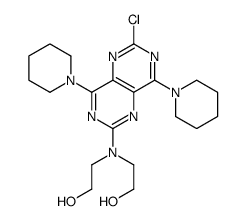
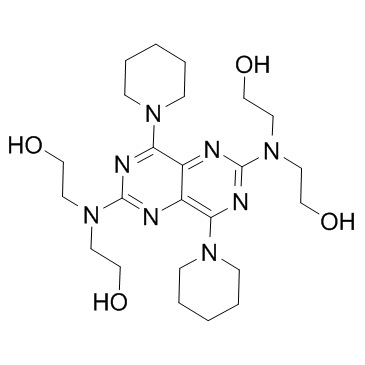
2,2',2'',2'''-[(4,8-Dipiperidin-1-ylpyrimido[5,4-d]pyrimidin-2,6-diyl)dinitrilo]tetraethanol
RA 8 Piroan EINECS 200-374-7 Dipyridamole Coridil Dypyridamole Apricor Anginal Natyl 2,2',2'',2'''-{[4,8-Di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl]dinitrilo}tetraethanol 2,2',2'',2'''-{[4,8-Di(1-piperidinyl)pyrimido[5,4-d]pyrimidine-2,6-diyl]dinitrilo}tetraethanol Corosan Coroxin MFCD00010555 Ethanol, 2,2',2'',2'''-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakis- Coribon |
| Description | Dipyridamole (Persantine) is a phosphodiesterase inhibitor that blocks uptake and metabolism of adenosine by erythrocytes and vascular endothelial cells.Target: Phosphodiesterase (PDE)Dipyridamole concentrations of 1 nmol/ml blood caused 90% inhibition of adenosine metabolism. Dipyridamole at therapeutic concentrations causes significant inhibition of adenosine metabolism in whole blood [1]. Dipyridamole has a dose-dependent inhibitory effect on thromboxane synthesis which was independent of aggregation. Dipyridamole also inhibited malonyldialdehyde production in response to both thrombin and arachidonic acid [2]. Dipyridamole enhances platelet inhibition by amplifying the signaling of the NO donor sodium nitroprusside. These data support the concept that enhancement of endothelium-dependent NO/cGMP-mediated signaling may be an important in vivo component of dipyridamole action [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 806.5±75.0 °C at 760 mmHg |
| Melting Point | 165-166ºC |
| Molecular Formula | C24H40N8O4 |
| Molecular Weight | 504.626 |
| Flash Point | 441.5±37.1 °C |
| Exact Mass | 504.317261 |
| PSA | 145.44000 |
| LogP | -1.22 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.670 |
| Storage condition | −20°C |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | KK7450000 |
| HS Code | 2933990090 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

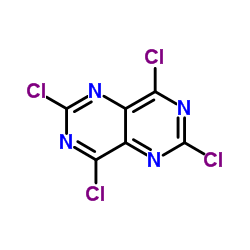
![2,6-DICHLORO-4,8-DIPIPERIDINOPYRIMIDINO[5,4-D]PYRIMIDINE structure](https://www.chemsrc.com/caspic/334/7139-02-8.png)