CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
OB1361000
-
CHEMICAL NAME :
-
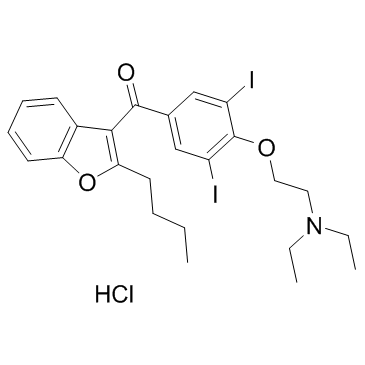
Ketone, 2-butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl, hydrochloride
-
CAS REGISTRY NUMBER :
-
19774-82-4
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
18
-
MOLECULAR FORMULA :
-
C25-H29-I2-N-O3.Cl-H
-
MOLECULAR WEIGHT :
-
681.81
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
3129 mg/kg/3Y-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - pleural thickening Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
SMJOAV Southern Medical Journal. (Southern Medical Assoc., POB 2446, Birmingham, AL 35205) V.1- 1908- Volume(issue)/page/year: 89,85,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
240 mg/kg/6W-I
-
TOXIC EFFECTS :
-
Skin and Appendages - hair
-
REFERENCE :
-
AIMDAP Archives of Internal Medicine. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1908- Volume(issue)/page/year: 155,1106,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1128 mg/kg/56W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - fibrosis, focal (pneumoconiosis) Lungs, Thorax, or Respiration - dyspnea Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
AJMEAZ American Journal of Medicine. (Technical Pub., 875 Third Ave., New York, NY 10022) V.1- 1946- Volume(issue)/page/year: 86,134,1989
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2480 mg/kg/43W-I
-
TOXIC EFFECTS :
-
Endocrine - thyroid weight (goiter)
-
REFERENCE :
-
AMSVAZ Acta Medica Scandinavica. (Almqvist & Wiksell, POB 45150, S-10430 Stockholm, Sweden) V.52-224, 1919-88. Volume(issue)/page/year: 221,219,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
171 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - respiratory stimulation Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
AHJOA2 American Heart Journal. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1925- Volume(issue)/page/year: 100,412,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
120 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Cardiac - arrhythmias (including changes in conduction)
-
REFERENCE :
-
HUTODJ Human Toxicology. (Macmillan Press Ltd., Houndmills, Basingstoke, Hants., RG 21 2XS, UK) V.1- 1981- Volume(issue)/page/year: 4,169,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
610 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
170 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
450 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,682,1992 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intratracheal
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
210 mg/kg/21D-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes Biochemical - Metabolism (Intermediary) - amino acids (including renal excretion)
-
REFERENCE :
-
TOXID9 Toxicologist. (Soc. of Toxicology, Inc., 475 Wolf Ledge Parkway, Akron, OH 44311) V.1- 1981- Volume(issue)/page/year: 13,154,1993 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
245 mg/kg
-
SEX/DURATION :
-
female 16-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 65,259,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - other measures of fertility Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 26,3871,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 26,3871,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 26,3871,1992
|