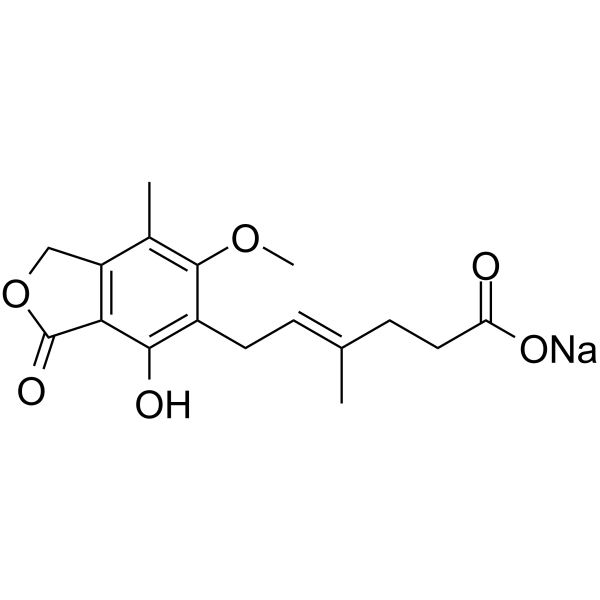
| In Vitro |
Mycophenolic acid sodium demonstrates antiviral effects against a wide range of RNA viruses including influenza, dengue virus, Zika virus, rotavirus, CCHFV, and hantavirus[1]. IMPDH is the rate-limiting enzyme in the de novo synthesis of guanosine nucleotides[2]. Mycophenolic acid (0.01-1 μM; 72 hours) sodium exhibits preferential antiproliferative activity against the endothelial cells and fibroblasts. Endothelial cells are most sensitive cells to Mycophenolic acid treatment with an IC50 <500 nM for antimitotic effects[2]. Fibroblasts are also prone to Mycophenolic acid-induced cell cycle inhibition but exhibit a higher IC50 (<1 μM) compared with endothelial cells. The two human tumor cell lines A549 non-small cell lung cancer cells and PC3 prostate cancer cells show intermediate sensitivity with an IC50 >1 μM. U87 glioblastoma cells are resistant against Mycophenolic acid sodium treatment up to 1 μM[2]. Mycophenolic acid (0.05-2 μM; 18 hours) sodium exhibits a dose-dependent down-regulation of HDAC2 and MYC, whereas up-regulates NDRG1[2]. Cell Proliferation Assay[2] Cell Line: Primary isolated human dermal microvascular endothelial cells (HDMVEC) , fibroblasts, U87 glioblastoma cells, PC3 prostate cancer cells, A549 non-small cell lung cancer cells. Concentration: 0.01, 0.1, 1 μM Incubation Time: 72 hours Result: Exhibited preferential antiproliferative activity against HDMVEC and fibroblasts. Whereas U87 glioblastoma cells were resistant to treatment, A549 non-small cell lung cancer and PC3 prostate cancer cells showed intermediate sensitivity. Western Blot Analysis[2] Cell Line: HDMVEC Concentration: 0, 0.05, 0.1, 0.5, 1, and 2 μM Incubation Time: 18 hours Result: Showed a dose-dependent regulation of HDAC2, MYC, and NDRG1.
|