68890-66-4
| Name | piroctone olamine |
|---|---|
| Synonyms |
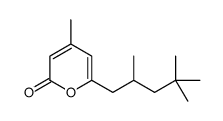
2(1H)-Pyridinone, 1-hydroxy-4-methyl-6-(2,4,4-trimethylpentyl)-, compd. with 2-aminoethanol (1:1)
1-Hydroxy-4-methyl-6-(2,4,4-trimethylpentyl)-2(1H)-pyridinone 2-Aminoethanol Salt 1-Hydroxy-4-methyl-6-(2,4,4-trimethylpentyl)pyridin-2(1H)-one - 2-aminoethanol (1:1) 1-Hydroxy-4-methyl-6-(2,4,4-trimethylpentyl)-2(1H)-pyridinone - 2-aminoethanol (1:1) MFCD01690792 octopirox Piroctone ethanolamine UNII:A4V5C6R9FB Piroctone olamine Piroctone ethanolamine salt 1-Hydroxy-4-methyl-6-(2,4,4-trimethylpentyl)-2(1H)-pyridinon--2-aminoethanol(1:1) EINECS 272-574-2 |
| Description | Piroctone olamine is a pyridine derivate. It is known to have a fungicidal effect. |
|---|---|
| Related Catalog | |
| Target |
Antifungal[1] |
| In Vitro | Piroctone olamine, the ethanolamine salt of the hydroxamic acid derivative Piroctone, is a hydroxypyridone anti-mycotic agent. Piroctone olamine penetrates the cell membrane and forms complexes with iron ions, inhibiting energy metabolism in mitochondria[1]. Piroctone olamine (PO) is an ethanolamine salt of the hydroxamic acid derivative Piroctone. All Candida strains show low minimum inhibitory concentrations (MICs) for Piroctone olamine (0.125-0.5 μg/mL) and Amphotericin B (AMB) (0.03-1 μg/mL)[2]. |
| In Vivo | This work aimed to evaluate the antifungal activity of Piroctone olamine in the treatment of intra-abdominal candidiasis in an experimental model using Swiss mice. The treatment with Piroctone olamine (0.5 mg/kg) is performed 72 h after infection by intraperitoneal administration. For comparison, a group of animals (n=6) is treated with Amphotericin B (0.5 mg/kg). The mycological diagnosis is made by collecting the liver, spleen and kidneys. Data regarding the fungal growth and mortality are analyzed statistically by Student’s t test and analysis of variance, with level of significance set at P<0.05. The difference in fungal growth scoring between the control group and the treatment groups (Piroctone olamine and Amphotericin B) is statistically significant (P<0.05)[2]. |
| Cell Assay | Ten different concentrations are used, ranging from 0.03 to 16 μg/mL of AMB and 0.125 to 64 μg/mL of FLZ. Piroctone olamine is diluted in DMSO to a stock solution concentration of 1600 μg/mL. The concentrations of Piroctone olamine (PO) range from 0.0625 to 32 μg/mL. The plates are incubated at 37°C and readings are taken after 24 and 48 h of incubation. Two control wells, free from other fungi and yeasts, are included in the assay. The readings are made visually for comparison against the growth in control wells. The minimum inhibitory concentration (MIC) is the lowest concentration capable of inhibiting visible growth of the isolates tested against the respective control well. Assays are performed in duplicate[2]. |
| Animal Admin | Mice[2] The swiss mice (n=6) are infected by intraperitoneal injection of 0.2 mL of C. albicans (107cells/ml in saline). The animals are observed daily for clinical signs and mortality for 14 days. The treatment with Piroctone olamine (0.5 mg/kg) is performed 72 h after infection by intraperitoneal administration. For comparison, a group of animals (n=6) is treated with Amphotericin B (0.5 mg/kg). The mycological diagnosis is made by collecting the liver, spleen and kidneys. Data regarding the fungal growth and mortality are analyzed statistically by Student’s t test and analysis of variance. |
| References |
| Density | 1.1 g/cm3 at 21.5 °C |
|---|---|
| Boiling Point | 344.1ºC at 760 mmHg |
| Melting Point | 130 - 135ºC |
| Molecular Formula | C16H30N2O3 |
| Molecular Weight | 298.421 |
| Flash Point | 161.9ºC |
| Exact Mass | 298.225647 |
| PSA | 88.48000 |
| LogP | 2.64650 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2.0 |
|
~% 
68890-66-4 |
| Literature: US3972888 A1, ; |
|
~% 
68890-66-4 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 31, # 8 a p. 1311 - 1316 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |