27740-01-8
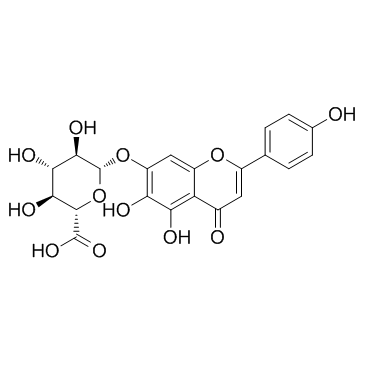
| Name | scutellarin |
|---|---|
| Synonyms |
5,6-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-7-yl-β-D-glucopyranosiduronic acid
Breviscapin Scutellarein-7-O-β-D-glucuronide Scutellarein-7β-D-glucuronoside Scutellarein-7β-D-glucuronide MFCD01861503 Scutellarein 7-O-β-D-glucuronide Breviscapin Scutellarin 7-(β-D-Glucopyranuronosyloxy)-5,6-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one Breviscapine Scutellarein 7-b-D-glucuronide 5,6-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl β-D-glucopyranosiduronic acid Scutellarein 7- 4H-1-Benzopyran-4-one, 7-(β-D-glucopyranuronosyloxy)-5,6-dihydroxy-2-(4-hydroxyphenyl)- Scutellarein-7-O-β-D-glucuronide |
| Description | Scutellarin, an active flavone isolated from Scutellaria baicalensis, can down-regulates the STAT3/Girdin/Akt signaling in HCC cells, and inhibits RANKL-mediated MAPK and NF-κB signaling pathway in osteoclasts. |
|---|---|
| Related Catalog | |
| Target |
STAT3 Akt |
| In Vitro | Scutellarin treatment significantly reduces HepG2 cell viability in a dose-dependent manner, and inhibits migration and invasion of HCC cells in vitro. Scutellarin treatment significantly reduces STAT3 and Girders of actin filaments (Girdin) expression, STAT3 and Akt phosphorylation in HCC cells. Introduction of STAT3 overexpression restores the scutellarin-downregulated Girdin expression, Akt activation, migration and invasion of HCC cells. Furthermore, induction of Girdin overexpression completely abrogates the inhibition of scutellarin on the Akt phosphorylation, migration and invasion of HCC cells. Scutellarin can inhibit HCC cell metastasis in vivo, and migration and invasion in vitro by down-regulating the STAT3/Girdin/Akt signaling[1]. Scutellarin selectively enhances Akt phosphorylation[2]. Scutellarin is a putative therapeutic agent as it has been found to not only suppress microglial activation thus ameliorating neuroinflammation, but also enhance astrocytic reaction. Acutellarin amplifies the astrocytic reaction by upregulating the expression of neurotrophic factors among others thus indicating its neuroprotective role. Remarkably, the effects of scutellarin on reactive astrocytes are mediated by activated microglia supporting a functional "cross-talk" between the two glial types[3]. Scutellarin can suppress RANKL-mediated osteoclastogenesis, the function of osteoclast bone resorption, and the expression levels of osteoclast-specific genes (tartrate-resistant acid phosphatase (TRAP), cathepsin K, c-Fos, NFATc1). Further investigation indicates that Scutellarin can inhibit RANKL-mediated MAPK and NF-κB signaling pathway, including JNK1/2, p38, ERK1/2, and IκBα phosphorylation[5]. |
| In Vivo | Scutellarin (50 mg/kg/day) significantly mitigates the lung and intrahepatic metastasis of HCC tumors in vivo. The numbers of the lung and intrahepatic metastatic tumors in the scutellarin-treated group are significantly less than that in the controls[1]. The rats treated with Scutellarin display a significant alleviation in neurobehavioral deficits compared to the SAH group. Scutellarin enhanced eNOS expression compared with SAH rats[4]. |
| Cell Assay | HepG2 cells (1×105/well) are cultured in 96-well plates and treated in triplicate with scutellarin at concentrations of 5, 10, 20, 30, and 100 μM or vehicle alone for 24 h. The cellular viability is tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and is expressed as a percentage of proliferation versus controls. |
| Animal Admin | To establish an orthotopic liver xenograft model, individual mice are anesthetized with isoflurane and a small incision is made in their abdomen. Individual mice are injected with 2×106 SK-Hep1 cells in 30 μL Matrigel into their left lobe of the liver. Twenty-four hours after orthotopic liver implantation, the mice are randomized and injected intraperitoneally with scutellarin (50 mg/kg/day) or vehicle (0.9% NaCl, normal saline) daily for 35 consecutive days (n=10 per group). Subsequently, the mice are sacrificed, and their lungs and livers are excised, fixed in 10% buffered formalin and paraffin-embedded for hematoxylin and eosin staining. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 891.6±65.0 °C at 760 mmHg |
| Molecular Formula | C21H18O12 |
| Molecular Weight | 462.360 |
| Flash Point | 314.9±27.8 °C |
| Exact Mass | 462.079834 |
| PSA | 207.35000 |
| LogP | -0.46 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.764 |
| Storage condition | 2-8°C |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
27740-01-8 |
| Literature: Ono, Eiichiro; Ruike, Miho; Iwashita, Takashi; Nomoto, Kyosuke; Fukui, Yuko Phytochemistry, 2010 , vol. 71, # 7 p. 726 - 735 |