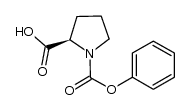
177834-92-3
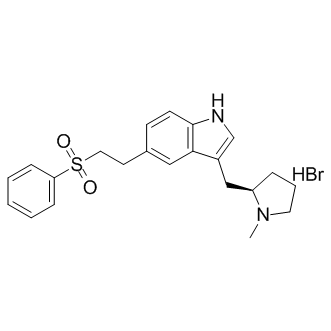
| Name | eletriptan hydrobromide |
|---|---|
| Synonyms |
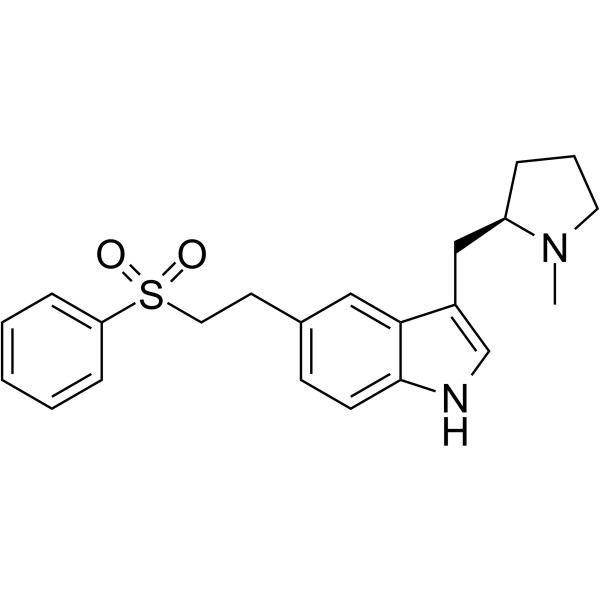
1H-indole, 3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-, monohydrobromide
Relert (R)-3-((1-Methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole monohydrobromide ELETRIPTAN HBR (R)-3-((1-methylpyrrolidin-2-yl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole hydrobromide 3-{[(2R)-1-méthylpyrrolidin-2-yl]méthyl}-5-[2-(phénylsulfonyl)éthyl]-1H-indole bromhydrate (R)-3-[(1-methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-indole hydrobromide salt 3-{[(2R)-1-Methylpyrrolidin-2-yl]methyl}-5-[2-(phenylsulfonyl)ethyl]-1H-indolhydrobromid 1H-Indole,3-(((2R)-1-methyl-2-pyrrolidinyl))methyl)-5-(2-(phenylsulfonyl)ethyl)-,monohydrobromide 3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-5-[2-(phenylsulfonyl)ethyl]-1H-indole hydrobromide 3-{[(2R)-1-Methylpyrrolidin-2-yl]methyl}-5-[2-(phenylsulfonyl)ethyl]-1H-indole hydrobromide (1:1) (R)-5-[2-(Benzenesulfonyl)ethyl]-3-[(N-Methylpyrrolidin-2-yl)Methyl]-1H-indole hydrobroMide 3-{[(2R)-1-Methyl-2-pyrrolidinyl]methyl}-5-[2-(phenylsulfonyl)ethyl]-1H-indole hydrobromide (1:1) 3-{[(2R)-1-Methylpyrrolidin-2-yl]methyl}-5-[2-(phenylsulfonyl)ethyl]-1H-indolhydrobromid(1:1) 3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-5-[2-(phenylsulfonyl) ethyl]-1H-indole hydrobromide (S)-3-((1-Methylpyrrolidin-2-yl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole 3-(((2r)-1-methyl-2-pyrrolidinyl))methyl)-5-(2-(phenylsulfonyl)ethyl)-1h-indole hydrobromide 1H-Indole, 3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-, hydrobromide (1:1) Eletriptan Base (R)-5-[2-(phenylsulfonyl)ethyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole hydrobromide Eletriptan hydrobromide (R)-5-(phenylsulfonylethyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole hydrobromide |
| Description | Eletriptan HBr is a selective 5-HT1B and 5-HT1D receptor agonist with Ki of 0.92 nM and 3.14 nM, respectively.IC50 value: 0.82 nM/3.14 nM (5-HT1B/5-HT1D, Ki) [1]Target: 5-HT1B/5-HT1D in vitro: [3H]Eletriptan has a total number of binding sites (Bmax) of 2478 fmol/mg and 1576 fmol/mg for 5-HT1B and 5-HT1D, respectively. [3H]Eletriptan has a significantly faster association rate (K(on) 0.249/min/nM) than [3H]sumatriptan (K(on) 0.024/min/nM) and a significantly slower off-rate (K(off) 0.027/min compared to 0.037/min for [3H]sumatriptan) [1]. Eletriptan induces concentration-dependent contractions of meningeal artery, coronary artery, and saphenous vein. The potency of Eletriptan is higher in meningeal artery than in coronary artery (86-fold) or saphenous vein (66-fold). The predicted contraction by Eletriptan (40 mg and 80 mg) and sumatriptan (100 mg) at free C(max) observed in clinical trials is similar in meningeal artery [2].in vivo: Eletriptan (<1000 mg/kg, i.v.) produces a dose-dependent reduction of carotid arterial blood flow in the anaesthetised dog. Eletriptan reduces coronary artery diameter with ED50 value of 63 mg/kg in the anaesthetised dog. Eletriptan (<300 mg/kg, i.v.) administered prior to electrical stimulation of the trigeminal ganglion produces a dose-related and complete inhibition of plasma protein extravasation in the dura mater rats. Eletriptan (100 mg/kg, i.v.) produces a complete inhibition of plasma protein extravasation in rat dura mater [3]. Iontophoretic ejection (50 nA) of Eletriptan suppresses the response in 75% of cells and causes an average suppression of cell firing of 42% in cats [4]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 633.9ºC at 760 mmHg |
|---|---|
| Melting Point | 169-171ºC |
| Molecular Formula | C22H27BrN2O2S |
| Molecular Weight | 463.43 |
| Flash Point | 337.2ºC |
| PSA | 61.55000 |
| LogP | 4.83970 |
| Vapour Pressure | 1.58E-16mmHg at 25°C |
| Storage condition | Desiccate at RT |
| Water Solubility | DMSO: >10mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

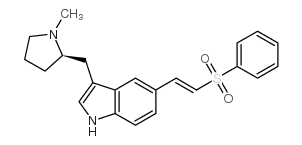
![1-[5-bromo-3-[[(2R)-1-methylpyrrolidin-2-yl]methyl]indol-1-yl]ethanone structure](https://www.chemsrc.com/caspic/288/205369-12-6.png)
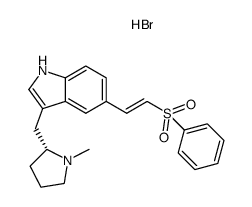
![5-[2-(benzenesulfonyl)ethyl]-3-[[(2R)-1-methylpyrrolidin-2-yl]methyl]-1H-indole,hydrate,hydrobromide structure](https://www.chemsrc.com/caspic/080/273211-28-2.png)