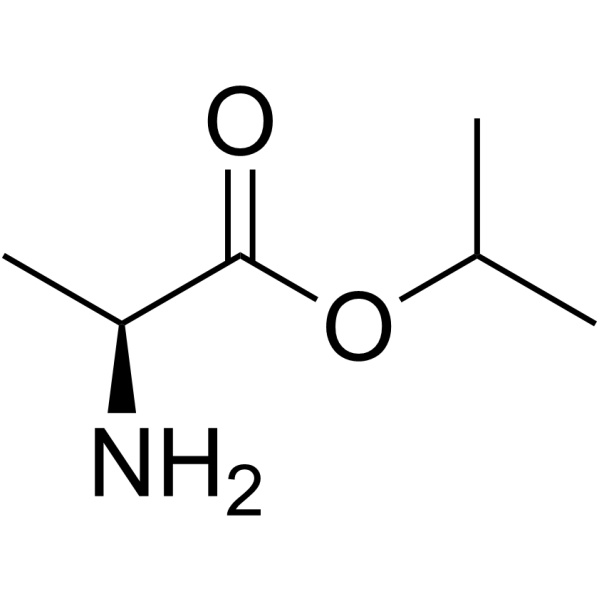
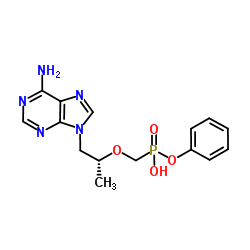
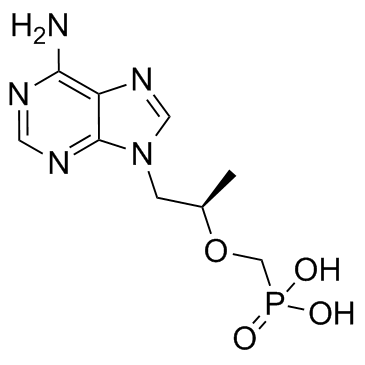
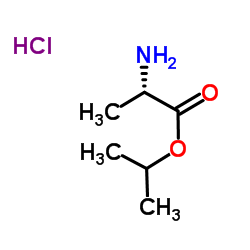
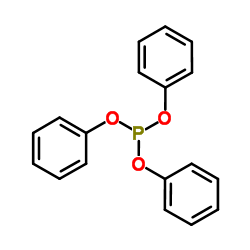
379270-37-8
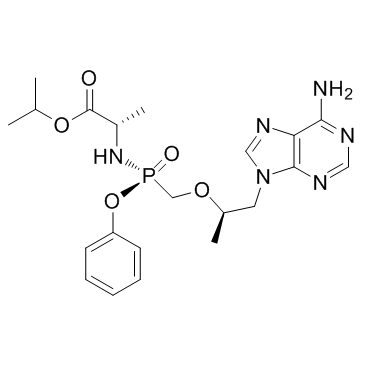
| Name | propan-2-yl (2S)-2-[[[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-phenoxyphosphoryl]amino]propanoate |
|---|---|
| Synonyms |
Isopropyl N-[(S)-({[(2R)-1-(6-amino-9H-purin-9-yl)-2-propanyl]oxy}methyl)(phenoxy)phosphoryl]-L-alaninate
GS7340 Sp-Tenofovir-phosphonamidate,phenyl,L-alanine isopropyl ester L-Alanine, N-[(S)-[(1S)-[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester Tenofovir alafenamide GS-7340 |
| Description | Tenofovir alafenamide (GS-7340) is an investigational oral prodrug of Tenofovir. Tenofovir is a HIV-1 nucleotide reverse transcriptase inhibitor. |
|---|---|
| Related Catalog | |
| Target |
HIV-1, NRTIs[1] |
| In Vitro | Tenofovir alafenamide antiviral activities are similar across all cell types, ranging from 5 to 7 nM, while the CC50 varies from 4.7 to 42 μM for MT-4 and MT-2 cells, respectively. The antiviral activity of TAF is evaluated against a panel of HIV-1 and HIV-2 isolates, including HIV-1 group M subtypes A to G, as well as group N and O isolates. Overall, for the 29 primary HIV-1 isolates tested in PBMCs, TAF EC50s range from 0.1 to 12 nM, with a mean EC50 of 3.5 nM compared to a mean EC50 of 11.8 nM for AZT, which is used as an internal control. For the HIV-2 isolates, the mean EC50s are 1.8 nM for TAF and 6.4 nM for AZT[2]. |
| In Vivo | Tenofovir alafenamide hemifumarate is an amidate prodrug of Tenofovir with good oral bioavailability and increases plasma stability compared to Tenofovir disoproxil fumarate (TDF)[1]. |
| References |
| Density | 1.39±0.1 g/cm3 |
|---|---|
| Boiling Point | 640.4±65.0 °C at 760 mmHg |
| Molecular Formula | C21H29N6O5P |
| Molecular Weight | 476.466 |
| Flash Point | 341.1±34.3 °C |
| Exact Mass | 476.193695 |
| PSA | 153.29000 |
| LogP | 2.20 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.630 |
| Water Solubility | Practically insoluble (0.04 g/L) (25 ºC) |
| Hazard Codes | Xi |
|---|
|
~% 
379270-37-8 |
| Literature: Chapman; Kernan; Prisbe; Rohloff; Sparacino; Terhorst; Yu Nucleosides, Nucleotides and Nucleic Acids, 2001 , vol. 20, # 4-7 p. 621 - 628 |
|
~% 
379270-37-8 |
| Literature: Chapman; Kernan; Prisbe; Rohloff; Sparacino; Terhorst; Yu Nucleosides, Nucleotides and Nucleic Acids, 2001 , vol. 20, # 4-7 p. 621 - 628 |
|
~% 
379270-37-8 |
| Literature: Colby, Denise A.; Martins, Andrew Anthony; Roberts, Benjamin James; Scott, Robert William; Solomon, Nicole S. Patent: US2013/90473 A1, 2013 ; |
|
~% 
379270-37-8 |
| Literature: Colby, Denise A.; Martins, Andrew Anthony; Roberts, Benjamin James; Scott, Robert William; Solomon, Nicole S. Patent: US2013/90473 A1, 2013 ; |
|
~% 
379270-37-8 |
| Literature: Colby, Denise A.; Martins, Andrew Anthony; Roberts, Benjamin James; Scott, Robert William; Solomon, Nicole S. Patent: US2013/90473 A1, 2013 ; |
|
~% 
379270-37-8 |
| Literature: Chapman; Kernan; Prisbe; Rohloff; Sparacino; Terhorst; Yu Nucleosides, Nucleotides and Nucleic Acids, 2001 , vol. 20, # 4-7 p. 621 - 628 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |