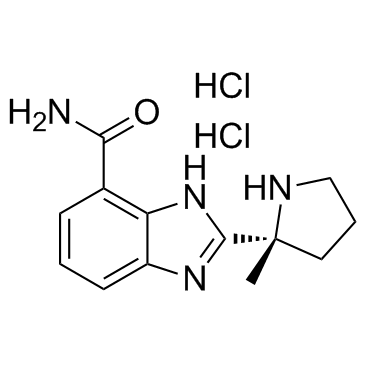
912445-05-7
| Name | 2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide,dihydrochloride |
|---|---|
| Synonyms |
2-[(2R)-2-Methyl-2-pyrrolidinyl]-1H-benzimidazole-4-carboxamide dihydrochloride
Veliparib dihydrochloride 1H-Benzimidazole-4-carboxamide, 2-[(2R)-2-methyl-2-pyrrolidinyl]-, hydrochloride (1:2) ABT-888 Veliparib Veliparib (dihydrochloride) |
| Description | Veliparib (dihydrochloride) is a potent inhibitor of PARP1 and PARP2 with Kis of 5.2 nM and 2.9 nM in cell-free assays, respectively. |
|---|---|
| Related Catalog | |
| Target |
PARP-2:2.9 nM (Ki) PARP-1:5.2 nM (Ki) |
| In Vitro | Veliparib is inactive to SIRT2 (>5 μM)[1]. Veliparib inhibits the PARP activity with EC50 of 2 nM in C41 cells[2]. Veliparib can decrease the PAR levels in both irradiated and nonirradiated H460 cells. Veliparib reduces clonogenic survival and inhibits DNA repair by PARP-1 inhibition in H460 cells. Veliparib increases apoptosis and autophagy in H460 cells when combination with radiation[3]. Veliparib inhibits PARP activity in H1299, DU145 and 22RV1 cells and the inhibition is independent of p53 function. Veliparib (10 μM) suppresses the surviving fraction (SF) by 43% in the clonogenic H1299 cells. Veliparib shows effective radiosensitivity in oxic H1299 cells. Veliparib can attenuate the SF of hypoxic-irradiated cells including H1299, DU145 and 22RV1[4]. |
| In Vivo | The oral bioavailability of Veliparib is 56%-92% in mice, SD rats, beagle dogs, and cynomolgus monkeys after oral administration[1]. Veliparib (25 mg/kg, i.p.) can improve tumor growth delay in a NCI-H460 xenograft model. Combination with radiation, veliparib decreases the tumor vessel formation[3]. Veliparib reduces intratumor PAR levels by more than 95% at a dose of 3 and 12.5 mg/kg in A375 and Colo829 xenograft models and the suppression can be maintained over time[4]. |
| Kinase Assay | PARP assays are conducted in a buffer containing 50 mM Tris (pH 8.0), 1 mM DTT, 1.5 μM [3H]NAD+ (1.6 μCi/mmol), 200 nM biotinylated histone H1, 200 nM slDNA, and 1 nM PARP-1 or 4 nM PARP-2 enzyme. Reactions are terminated with 1.5 mM benzamide, transferred to streptavidin Flash plates, and counted using a TopCount microplate scintillation counter. |
| Animal Admin | For B16F10 syngeneic studies, 6×104 cells are mixed with 50% Matrigel and inoculated by s.c. injection into the flank of 6- to 8-week-old female C57BL/6 mice (20 g). For cisplatin efficacy studies, female nude mice are implanted s.c. by trocar with fragments (20-30 mm3) of human tumors harvested from s.c. grown tumors in nude mice hosts. For the carboplatin and MX-1 cyclophosphamide studies, female scid mice are inoculated with 200 μL of a 1:10 dilution of tumor brei in 45% Matrigel and 45% Spinner MEM. For these established tumor studies, tumors are allowed to grow to the indicated size and then randomized to therapy groups. For DOHH-2 xenograft studies, 1×106 cells are mixed with 50% Matrigel and inoculated by s.c. injection into the flank of male scid mice. Veliparib is delivered by either oral route or continuous infusion using s.c. placement of 14-day Alzet OMP model 2002 in a vehicle containing 0.9% NaCl adjusted to pH 4.0. The OMP delivers at a rate of 12 μL daily and Veliparib doses are calculated accordingly. Temozolomide, cisplatin, carboplatin, and cyclophosphamide are formulated according to the manufacturers' recommendations. |
| References |
| Molecular Formula | C13H18Cl2N4O |
|---|---|
| Molecular Weight | 317.214 |
| Exact Mass | 316.085754 |
| PSA | 4.07740 |
| LogP | 3.00000 |
| Storage condition | 2-8℃ |