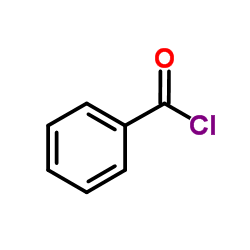
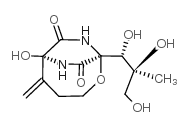
37134-40-0
| Name | [(2S,3S)-2,3-dihydroxy-3-[(1S)-1-hydroxy-2-methylidene-7,9-dioxo-5-oxa-8,10-diazabicyclo[4.2.2]decan-6-yl]-2-methylpropyl] benzoate |
|---|---|
| Synonyms |
2-Oxa-7,9-diazabicyclo[4.2.2]decane-8,10-dione, 1-[(1S,2S)-3-(benzoyloxy)-1,2-dihydroxy-2-methylpropyl]-6-hydroxy-5-methylene-, (1S,6R)-
Bicyclomycin,3'-benzoate O3'-benzoyl-bicyclomycin Bicyclomycin benzoate Bicyclomycin-benzylat (2S,3S)-2,3-Dihydroxy-3-[(1S,6R)-6-hydroxy-5-methylene-8,10-dioxo-2-oxa-7,9-diazabicyclo[4.2.2]dec-1-yl]-2-methylpropyl benzoate |
| Description | Bicyclomycin benzoate is an antibiotic exhibiting activity against a broad spectrum of Gram-negative bacteria and against the Gram-positive bacterium. |
|---|---|
| Related Catalog | |
| In Vitro | The primary action of bicyclomycin is due to interference with the biosynthesis of lipoprotein and its assembly to peptidoglycan in the cell envelope of E. coli. At the lethal level, bicyclomycin is shown to inhibit the synthesis of RNA and protein in the growing cells of E. coli 15 THU[1]. Bicyclomycin targets the rho transcription termination factor in Escherichia coli. Bicyclomycin is a modest rho inhibitor, can disrupt the rho molecular machinery thereby leading to a catastrophic effect caused by the untimely overproduction of proteins not normally expressed constitutively, thus leading to a toxic effect on the cells[2]. The inhibition of rho poly(C)-stimulated hydrolysis of ATP by bicyclomycin has been found to proceed by a non-competitive, reversible pathway with respect to ATP (Ki=20 μM)[3]. |
| In Vivo | Bicyclomycin has low excretion rate after a single intramuscular dose of 50 mg/kg in rats. Bicyclomycin is well distributed in various tissues, and the highest concentration is observed in the kidney at 100 mg/kg[4]. |
| Kinase Assay | ATPase activity assays are carried out with rho (100 ng) except that either bicyclomycin (0–60 μM) or dihydrobicyclomycin (0–90 μM) is added to the reaction solution. The samples are preheated to 32°C (2.5 min), and the reaction is initiated by the addition of ATP (9.1–100 μM) and [g-32P]ATP (0.5mCi) to the side of the tube, briefly vortexed, centrifuged (2 s), and returns to the water bath. Aliquots (1 mL) are removed at five time points (15, 30, 45, 60, and 75 s), and spotted on Baker-Flex cellulosePEI TLC plates. The rates of reaction are determined by measuring the relative amount of radiolabeled inorganic phosphate and ATP and then plotting the amount of ATP hydrolyzed versus time. The initial rates for each ATP concentration plus or minus inhibitors are plotted as double reciprocal plots[3]. |
| Animal Admin | Rats: A total of 25 SD male rats receives intramuscular administration of bicyclomycin at a single dose of 50mg/kg. Blood samples are taken by heart-puncture from 5 rats each at 0.5, 1, 2, 3, and 5 hours after the administration[4]. Mice: A total of 40 ICR male mice receives intramuscular administration of bicyclomycin at a single dose of 50mg/kg. Eight mice each are bled at 5, 10, 20, 30 and 60 minutes after administration by heart-puncture with heparin[4]. Rabbits: Five rabbits and 5 dogs are given bicyclomycin intramuscularly at a single dose of 50 mg/kg and blood samples are withdrawn from these animals at similar intervals. The samples are allowed to clot and sera are separated for assay by the cylinder plate method[4]. |
| References |
[1]. Tanaka N, et al. Mechanism of action of bicyclomycin. J Antibiot (Tokyo). 1976 Feb;29(2):155-68. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 788.6±60.0 °C at 760 mmHg |
| Molecular Formula | C19H22N2O8 |
| Molecular Weight | 406.387 |
| Flash Point | 430.7±32.9 °C |
| Exact Mass | 406.137604 |
| PSA | 161.40000 |
| LogP | 1.87 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.640 |
| Storage condition | 2-8℃ |
|
~% 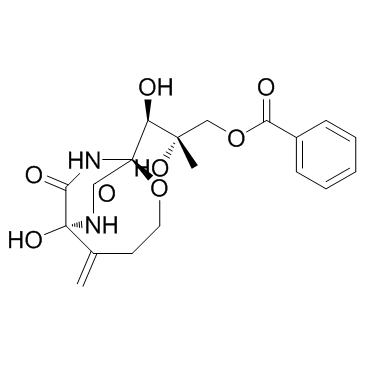
37134-40-0 |
| Literature: Kamiya; Maeno; Hashimoto; Mine The Journal of antibiotics, 1972 , vol. 25, # 10 p. 576 - 581 |