154-17-6
| Name | 2-Deoxy-D-Glucose |
|---|---|
| Synonyms |
2-DEOXY-D-ARABINOHEXOSE
D-arabino-Hexose, 2-deoxy- 2-Deoxy-D-arabino-hexose 2-Deoxy-D-glucose 2-deoxy-d-arabino-hexos MFCD00151328 D-Arabino-2-deoxyhexose EINECS 205-823-0 |
| Description | 2-Deoxy-D-glucose is a glucose analog that acts as a competitive inhibitor of glucose metabolism, inhibiting glycolysis via its actions on hexokinase. |
|---|---|
| Related Catalog | |
| In Vitro | 2-Deoxy-D-glucose (2-DG, 4, 8, or 16 mM) significantly reduces the level of ATP in MCF-7 cells in a dose- and time-dependent manner that paralleles the effects of 2-DG on cell growth. The levels of phosphorylated Akt are significantly decreased, whereas the levels of phosphorylated AMPK and Sirt-1 are significantly increased in MCF-7 cells exposed to 2-Deoxy-D-glucose at 4, 8, or 16 mM for 1, 3, or 5 days in a dose- and time-dependent manner[1]. 2-DG treatment increases the levels of pentose phosphate pathway (PPP) metabolites and augments the generation of NADPH by glucose-6-phosphate dehydrogenase. An increase in NADPH and upregulation of glutathione synthetase expression resultes in the increase in the reduced form of glutathione by 2-DG in NB4 cells[3]. |
| In Vivo | 2-Deoxy-D-glucose (0.03%, w/w) causes a 7% decrease in final weight that is statistically significant, and delayes the appearance of palpable mammary carcinomas[1]. 2-Deoxy-D-glucose (3 mmol/kg, i.v.) is decreased in a dose-dependent manner by insulin in rat muscle[2]. |
| Cell Assay | The effect of 2-DG on cell growth is determined by evaluating the number of adherent cells. Briefly, MCF-7 cells are plated at 3×104 cells per well in flat-bottomed 96-well plates in 100 μL of culture medium under the culture conditions. After 24 hours, cells are fed with fresh medium including 2-Deoxy-D-glucose at doses of 0, 4, 8, or 16 mM. At days 1, 3, and 5 after 2-Deoxy-D-glucose exposure, cells are fixed with 1% glutaraldehyde, replaced with PBS, and stored at 4°C. At the end of an experiment, all of the plates are stained with 0.02% aqueous crystal violet for 30 minutes and rinsed with deionized water. After redissolving the bound crystal violet in 70% ethanol, the absorbance is determined at 590 nm using a SPECTRA MAX PLUS Microplate Spectrophotometer System. |
| Animal Admin | At 21 days of age, rats are injected with 50 mg 1-methyl-1-nitrosourea per kilogram of body weight (i.p.). Rats are housed two per cage in solid-bottomed polycarbonate cages equipped with a food cup. Six days following carcinogen injection, all rats are randomized into one of three groups, 30 rats per group, and are fed ad libitum AIN-93G diet containing 0.0%, 0.02%, or 0.03% (w/w) 2-Deoxy-D-glucose (2-DG) for 5 weeks. Animal rooms are maintained at 22±1°C with 50% relative humidity and a 12-hour light/12-hour dark cycle. Rats are weighed thrice per week and are palpated for detection of mammary tumors twice per week starting from 19 days postcarcinogen. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.7±45.0 °C at 760 mmHg |
| Melting Point | 146-147ºC |
| Molecular Formula | C6H12O5 |
| Molecular Weight | 164.156 |
| Flash Point | 244.1±25.2 °C |
| Exact Mass | 164.068466 |
| PSA | 97.99000 |
| LogP | -3.07 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.534 |
| Storage condition | 2~8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MQ3325000 |
| HS Code | 2912491000 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2912491000 |
|---|---|
| Summary | 2912491000. other aldehyde-alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
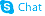





![[2-oxo-2-(1H-pyrrol-2-yl)ethyl] benzoate structure](https://www.chemsrc.com/caspic/122/5729-75-9.png)