29094-61-9
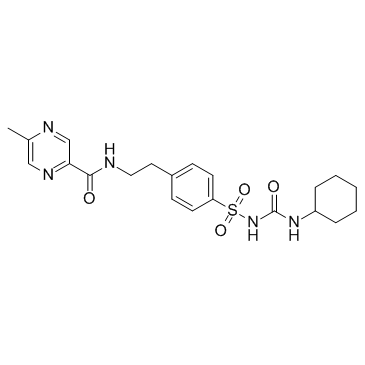
| Name | glipizide |
|---|---|
| Synonyms |
EINECS 249-427-6
N-[2-(4-{[(E)-(Cyclohexylimino)(hydroxy)methyl]sulfamoyl}phenyl)ethyl]-5-methyl-2-pyrazinecarboximidic acid Glican Ozidia N-(2-{4-[(cyclohexylcarbamoyl)sulfamoyl]phényl}éthyl)-5-méthylpyrazine-2-carboxamide Glipid N-(2-{4-[(Cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-5-methyl-2-pyrazinecarboxamide k4024 N-(2-{4-[(Cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-5-methylpyrazin-2-carboxamid N-(2-{4-[(cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-5-methylpyrazine-2-carboxamide exylurea 2-Pyrazinecarboxamide, N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-5-methyl- Pyrazinecarboxamide, N-(2-(4-((((cyclohexylamino)carbonyl)amino)sulfonyl)phenyl)ethyl)-5-methyl- Glucotrol Glipizide MFCD00072159 N-[4-(3-Cyclohexylureidosulfonyl)phenethyl]-5-methyl-2-pyrazinecarboxamide Mindiab Digrin Glibenese Glidiab 1-Cyclohexyl-3-{4-[2-(5-methylpyrazine-2-carboxamido)ethyl]phenylsulfonyl}urea tk1320 Aldiab 2-Pyrazinecarboximidic acid, N-[2-[4-[[[(E)-(cyclohexylimino)hydroxymethyl]amino]sulfonyl]phenyl]ethyl]-5-methyl- Melizide |
| Description | Glipizide(K 4024; CP 2872) is used to treat high blood sugar levels caused by a type of diabetes mellitus called type 2 diabetes.Target: Potassium ChannelGlipizide is an oral rapid- and short-acting anti-diabetic drug from the sulfonylurea class. It is classified as a second generation sulfonylurea, which means that it undergoes enterohepatic circulation. Mechanism of action is produced by blocking potassium channels in the beta cells of the islets of Langerhans. By partially blocking the potassium channels, the cell remains depolarized, increasing the time the cell spends in the calcium release stage, which results in signaling leading to calcium influx. The increase in calcium will initiate more insulin release from each beta cell. Sulfonylureas may also cause the decrease of serum glucagon and potentiate the action of insulin at the extrapancreatic tissues [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 676.0±65.0 °C at 760 mmHg |
| Melting Point | 208-209°C |
| Molecular Formula | C21H27N5O4S |
| Molecular Weight | 445.535 |
| Flash Point | 362.6±34.3 °C |
| Exact Mass | 445.178375 |
| PSA | 138.53000 |
| LogP | 3.37 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.654 |
| Storage condition | -20°C Freezer |
| Water Solubility | methanol: 1.9 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant;Xn: Harmful; |
| Risk Phrases | R21 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YS7640000 |
| HS Code | 2935009090 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |