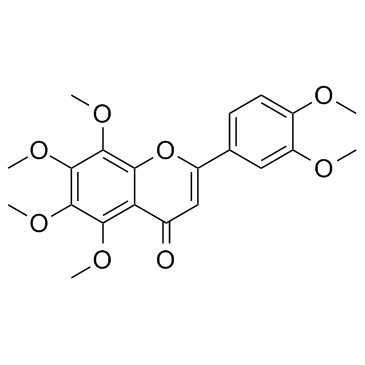
1176-88-1
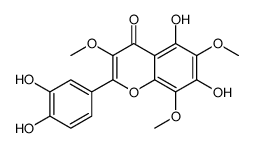
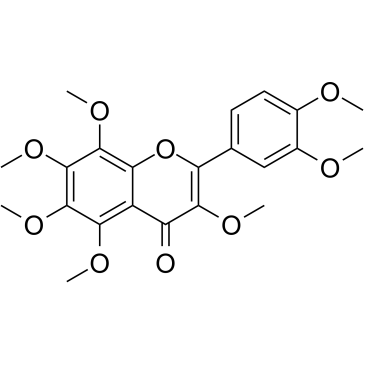
| Name | 2-(3,4-dimethoxyphenyl)-5-hydroxy-3,6,7,8-tetramethoxychromen-4-one |
|---|---|
| Synonyms |
2-(3,4-dimethoxy-phenyl)-5-hydroxy-3,6,7,8-tetramethoxy-chromen-4-one
HMS2267M18 5-hydroxy-3,6,7,8,3',4'-hexamethoxyflavone |
| Description | 5-OH-HxMF is a hydroxylated polymethoxyflavone that has anti-inflammatory, anticancer, neurotrophic and neuroprotective activities[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | 5-OH-HxMF(5-20 µM;48 小时)有效诱导 PC12 神经突生长,并伴有神经元分化标记蛋白生长相关蛋白 43(GAP-43)的表达[1] 。 5-OH-HxMF(20 µM;0-120 分钟)导致环 AMP 反应元件结合蛋白 (CREB) 磷酸化、c-fos 基因表达和 CRE 介导的转录增强[1]。 5-OH-HxMF 显着减少一氧化氮和前列腺素 E2 的产生,并下调脂多糖 (LPS) 刺激的 RAW 264.7 细胞中的诱导型一氧化氮合酶 (iNOS) 和 COX-2 表达。5-OH-HxMF 抑制促炎细胞因子的释放,例如肿瘤坏死因子-α 和 IL-1β,并降低转录水平。5-OH-HxMF 显着抑制 LPS 诱导的 NF-κB 从细胞质向细胞核的易位,这与抑制性 IκBα 降解的消除和随后核 p65 水平的降低有关[2]。 5-OH-HxMF 在人白血病细胞中抑制细胞生长并诱导细胞凋亡[3]。PC12 cells10 µM, 20 µM24 hPromoted GAP-43 expression in PC12 cells.PC12 cells20 µM0 min, 30 min, 60 min or 120 minStimulated phosphorylation of CREB in PC12 cells. Cell Viability Assay[1] Cell Line: PC12 cells Concentration: 5 µM, 10 µM, 20 µM Incubation Time: 48 h Result: Significantly evoked a dose-dependent increase on neurite outgrowth. Western Blot Analysis[1] Cell Line: PC12 cells Concentration: 5 µM, 10 µM, 20 µM Incubation Time: 24 h Result: Promoted GAP-43 expression in PC12 cells. Western Blot Analysis[1] Cell Line: PC12 cells Concentration: 20 µM Incubation Time: 0 min, 30 min, 60 min or 120 min Result: Stimulated phosphorylation of CREB in PC12 cells. |
| In Vivo | 5-OH-HxMF(局部处理;每周两次;20 周;200 μL 丙酮中 1 和 3 μmol)是一种有效的抗肿瘤剂,其抑制作用是通过下调炎症 iNOS 和 COX-2 基因表达在小鼠皮肤中[3]。 Animal Model: Female ICR mice treated with 12-O-tetradecanoylphorbol-13-acetate (TPA)[3]. Dosage: 1 and 3 μmol in 200 μL acetone Administration: Topically treated; twice a week; for 20 weeks Result: Significantly inhibited TPA-induced mouse skin inflammation by decreasing inflammatory parameters. |
| References |
| Density | 1.37g/cm3 |
|---|---|
| Boiling Point | 631.7ºC at 760mmHg |
| Molecular Formula | C21H22O9 |
| Molecular Weight | 418.39400 |
| Flash Point | 221.4ºC |
| Exact Mass | 418.12600 |
| PSA | 105.82000 |
| LogP | 3.21720 |
| Vapour Pressure | 1.49E-16mmHg at 25°C |
| Index of Refraction | 1.599 |
| HS Code | 2914509090 |
|---|
|
~% 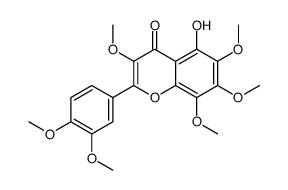
1176-88-1 |
| Literature: Phytochemistry (Elsevier), , vol. 24, # 4 p. 835 - 848 |
|
~% 
1176-88-1 |
| Literature: Proceedings - Indian Academy of Sciences, Section A, , # 23 p. 192,200 |
|
Detail
|
| Literature: WO2007/83263 A1, ; Page/Page column 6-8 ; |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |