701213-36-7
| Name | 1-(4-benzoylpiperazin-1-yl)-2-[4-methoxy-7-(3-methyl-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]ethane-1,2-dione |
|---|---|
| Synonyms |
UNII-4B6J53W8N3
1,2-Ethanedione, 1-(4-benzoyl-1-piperazinyl)-2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]- 1-(4-benzoylpiperazin-1-yl)-2-[4-methoxy-7-(3-methyl-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]ethane-1,2-dione 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine 1-(4-Benzoyl-1-piperazinyl)-2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-ethanedione BMS-626529 Piperazine, 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]- Piperazine,1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl] Temsavir CS-0938 1-(4-benzoyl-piperazin-1-yl)-2-[4-methoxy-7-(3-methyl-[1,2,4]triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-ethane-1,2-dione BMS 626529 |
| Description | BMS-626529 is a novel attachment inhibitor that targets HIV-1 gp120 and prevents its binding to CD4+ T cells. |
|---|---|
| Related Catalog | |
| Target |
HIV-1[1] |
| In Vitro | BMS-626529 has half-maximal effective concentration (EC50) values of <10 nM against the vast majority of viral isolates. BMS-626529 exhibits an average EC50 against LAI virus of 0.7±0.4 nM. BMS-626529 exhibits an EC50 of 0.01 nM against the most susceptible virus and an EC50 of >2,000 nM against the least susceptible virus. The cytotoxicity profile of BMS-626529 is examined in several cell types from different human tissues. CC50 values of >200 μM are observed in MT-2 (T lymphocytes), HEK293 (kidney), HEp-2 (larynx), HepG2 (liver), HeLa (cervix), HCT116 (colorectal), MCF-7 (breast), SK-N-MC (neuroepithelium), HOS (bone), H292 (lung), and MDBK (bovine kidney) cells measured after 3 or 6 days in culture. CC50 values of 105 and 192 μM are obtained in the T-cell line PM1 and in PBMCs, respectively, following 6 days in culture. These results show that BMS-626529 exhibits low cytotoxicity in cell culture[1]. BMS-626529 exhibits a broad spectrum of antiviral activity against a panel of clinical isolates, with a 50% inhibitory concentration (IC50) ranging from subnanomolar levels to >0.1 µM[2]. |
| Kinase Assay | Micro BioSpin 6 columns are used to measure the binding of [3H]BMS-488043 or [3H]BMS-626529 to gp120. Binding solutions (30 μL) containing 25 mM Tris-HCl (pH 7.5), 125 mM NaCl, 50 nM gp120JRFL, and serial dilutions of [3H]BMS-488043 or [3H]BMS-626529 are allowed to equilibrate and then adsorbed to a MicroBioSpin 6 column. The column is centrifuged (~14,000 rpm) for 5 min, the eluent is collected, and radioactivity is determined with a scintillation counter. To measure dissociative kinetics, 150 nM [3H]BMS-626529 or 90 nM [3H]BMS-488043 is incubated with 60 nM gp120 at ambient temperature for 1 h to achieve equilibrium binding, and then a large molar excess (14-fold) of soluble CD4 protein is added to drive dissociation. Aliquots are taken at the indicated time intervals, adsorbed to a spin column, and centrifuged, and the radioactivity in the eluent is quantitated. Comparison of the tritium signal from parallel samples with and without the soluble CD4 challenge allowed for the determination of the percent compound bound[1]. |
| Cell Assay | Cytotoxicity assays are performed in the presence of serially diluted BMS-626529 for up to 6 days, and cell viability is quantitated using an XTT assay. To determine CC50 values (concentration of drug required to kill 50% of cells), laboratory-adapted peripheral blood mononuclear cells (PBMCs) are initially plated at a density of 0.1×106 cells/mL. In the absence of compounds, the cell densities typically reach 1×106 to 1.2×106/mL after 6 days[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 787.6±70.0 °C at 760 mmHg |
| Molecular Formula | C24H23N7O4 |
| Molecular Weight | 473.484 |
| Flash Point | 430.1±35.7 °C |
| Exact Mass | 473.181152 |
| PSA | 126.31000 |
| LogP | -1.49 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.722 |
|
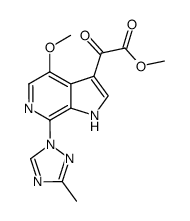
~81% 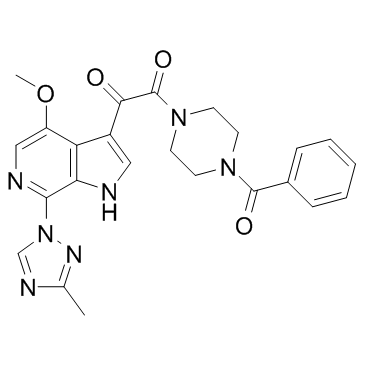
701213-36-7 |
| Literature: Bristol-Myers Squibb Company Patent: US2006/293304 A1, 2006 ; Location in patent: Page/Page column 32-33 ; |
|
~75% 
701213-36-7 |
| Literature: Ueda, Yasutsugu; Connolly, Timothy P.; Kadow, John F.; Meanwell, Nicholas A.; Wang, Tao; Chen, Chung-Pin H.; Yeung, Kap-Sun; Zhang, Zhongxing; Leahy, David Kenneth; Pack, Shawn K.; Soundararajan, Nachimuthu; Sirard, Pierre; Levesque, Kathia; Thoraval, Dominique Patent: US2005/209246 A1, 2005 ; Location in patent: Page/Page column 29 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |