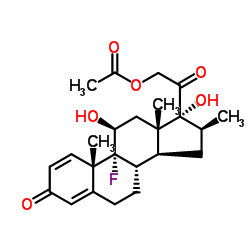
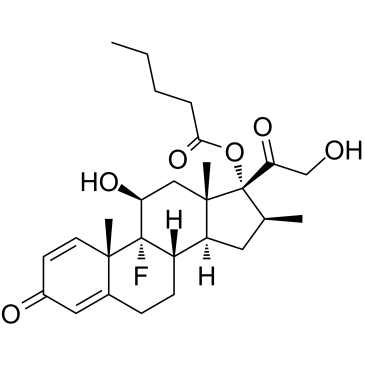
378-44-9
| Name | betamethasone |
|---|---|
| Synonyms |
16β-Methyl-1,4-pregnadiene-9α-fluoro-11β,17α,21-triol-3,20-dione
Bifas Betasolon (8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11,17-dihydroxy-17-(hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one Betadexamethasone Celestene Flubenisolone Visubeta 9A-FLUORO-16β-METHYLPREDNISOLONE 9α-Fluoro-11β,17α,21-trihydroxy-16β-methyl-1,4-pregnadiene-3,20-dione b-Methasone alcohol Bebate EINECS 206-825-4 16b-Methyl-9a-fluoroprednisolone Celestone Pregna-1,4-diene-3,20-dione, 9-fluoro-11β,17,21-trihydroxy-16β-methyl- pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11b,16b)- Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11β,16β)- betamethazone (11b,16b)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 9α-Fluoro-16β-methylprednisolone Betametasona [INN-Spanish] Betnelan b-Methasone β-Methasone Betametasone [DCIT] DERMABET Pertene UNII-9842X06Q6M β-Methasone alcohol MFCD00062969 Betsolan (11β,16β)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 4-08-00-03501 (Beilstein Handbook Reference) Diprolene Bedifos Becort Cidoten Bentelan β-Corlan b-Corlan 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione 9a-Fluoro-16b-methylprednisolone Betamethasone Solutiom 6beta) Betamethasone Betalon 16b-Methyl-9a-fluoro-D1-hydrocortisone |
| Description | Betamethasone is a glucocorticoid steroid with anti-inflammatory and immunosuppressive properties.Target: Glucocorticoid ReceptorBetamethasone is a potent glucocorticoid steroid with anti-inflammatory and immunosuppressive properties. Unlike other drugs with these effects, betamethasone does not cause water retention. The median (range) IC50 value for betamethasone butyrate propionate evaluated in the streptococcal pyrogenic enterotoxin A-stimulated peripheral-blood mononuclear cells was 291.6 (0.001-1171.5) ng/ml, which was significantly higher than the value 0.072 (0.01-222.5) ng/ml found in concanavalin A-stimulated peripheral-blood mononuclear cells (P=0.0245) [1]. Children exposed prenatally to betamethasone (n = 121) did not differ in systolic or diastolic blood pressure from children exposed to placebo (n = 102) (mean difference: systolic: -1.6 mm Hg; 95% confidence interval: -4.1 to 0.8 mm Hg; diastolic: -0.3 mm Hg; 95% confidence interval: -2.5 to 1.8 mm Hg) [2]. Intra-articular corticosteroid injection of 6 mg of betamethasone acetate/betamethasone sodium phosphate at the knee joint was not significantly associated with SAI at the time points tested [3].Clinical indications: Dermatitis; Discoid lupus erythematosus; Eczema; Lichen; Prurigo; PsoriasisToxicity: Symptoms of overdose include burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 568.2±50.0 °C at 760 mmHg |
| Melting Point | 235-237°C |
| Molecular Formula | C22H29FO5 |
| Molecular Weight | 392.461 |
| Flash Point | 297.5±30.1 °C |
| Exact Mass | 392.199890 |
| PSA | 94.83000 |
| LogP | 1.87 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.592 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360-H373 |
| Precautionary Statements | P201-P308 + P313 |
| Target Organs | Endocrine system, Kidney, Liver |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi,Xn |
| Risk Phrases | R40 |
| Safety Phrases | 22-36 |
| RIDADR | NONH for all modes of transport |
| RTECS | TU4000000 |
| HS Code | 2937229000 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
| HS Code | 2937229000 |
|---|