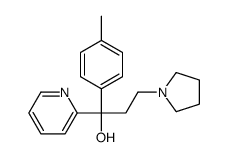
486-12-4
| Name | triprolidine |
|---|---|
| Synonyms |
MFCD00038040
(e)-pyridin Triprolidin (E)-2-[3-(1-Pyrrolidinyl)-1-p-toluenepropenyl]pyridine (E)-1-(4-methylphenyl)-1-(2-pyridyl)-3-pyrrolidino prop-1-ene trans-2-[3-(1-Pyrrolidinyl)-1-p-tolypropenyl]pyridine trans-1-(2-Pyridyl)-3-pyrrolidino-1-p-tolylprop-1-ene trans-2-[3-(1-pyrrolidinyl)-1-p-tolylpropenyl]pyridine EINECS 207-627-0 Triprolidine Tripyrolidine 1t-[2]Pyridyl-3-pyrrolidino-1c-p-tolyl-propen (E)-1-(2-pyridyl)-3-pyrrolidin-1-yl-1-p-tolylpropene 1t-[2]pyridyl-3-pyrrolidino-1c-p-tolyl-propene (E)-2-[1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]pyridine |
| Description | Triprolidine is an orally active H1R Antagonist antagonist. Triprolidine has the function of spinal cord motor and sensory block. Triprolidine can be used for the research of allergic rhinitis[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Triprolidine (maturing human dendritic cells) can antagonist histamine H1 and decreases the expression of CD45[1]. |
| In Vivo | Triprolidine (292.81-1467.20 μg/kg; i.p.; Male Sprague-Dawley rat) produces a dose-dependent effect of spinal motor and sensory block in rats[2]. Animal Model: Male Sprague-Dawley rat (300-350 g)[2] Dosage: 292.81, 488.02, 733.60, 1098.83 and 1467.20 μg/kg Administration: Intrathecal injection Result: Elicited spinal block in a dose-dependent. |
| References |
| Density | 1.061 g/cm3 |
|---|---|
| Boiling Point | 435.4ºC at 760 mmHg |
| Melting Point | 126-130°C |
| Molecular Formula | C19H22N2 |
| Molecular Weight | 278.39100 |
| Flash Point | 217.1ºC |
| Exact Mass | 278.17800 |
| PSA | 16.13000 |
| LogP | 3.85540 |
| Vapour Pressure | 6.16E-09mmHg at 25°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S36/37 |
|---|
|
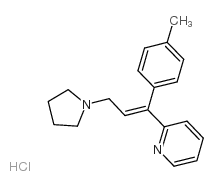
~82% 
486-12-4 |
| Literature: HIKAL LIMITED Patent: WO2009/84035 A1, 2009 ; Location in patent: Page/Page column 5; 9-10 ; |
| Precursor 1 | |
|---|---|
| DownStream 1 | |