4460-86-0
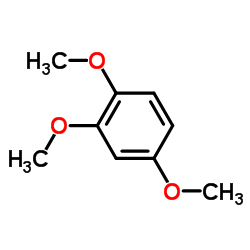
| Name | 2,4,5-Trimethoxybenzaldehyde |
|---|---|
| Synonyms |
2,4,5-Trimethoxybenzolcarbaldehyd
2,4,5-Trimethoxy 2,4,5-Trimethoxybenzaldehyde ASARONALDEHYDE EINECS 224-713-3 2,4,5-Trimethoxybenz Azarylaldehyde 2,4,5-Trimethoxy benzaldehyde ASARALDEHYDE 2,4,5-tris-methoxy benzaldehyde Gazarin Asarylaldehyde 2,4,5-trimethyl ether MFCD00003312 Benzaldehyde, 2,4,5-trimethoxy- |
| Description | Asarylaldehyde is a natural COX-2 inhibitor, which isolated from carrot (Daucus carota L.) seeds significantly inhibits cyclooxygenase II (COX-2) activity at IC50 value 100 μg/mL. |
|---|---|
| Related Catalog | |
| Target |
COX-2 |
| In Vitro | Asarylaldehyde (2,4,5-TMBA) is a natural COX-2 inhibitor, which isolated from carrot (Daucus carota L.) seeds significantly inhibits cyclooxygenase II (COX-2) activity at the concentration of 100 μg/mL compared to three commercial nonsteroidal anti-inflammatory drugs Aspinin, Ibuprofen, and Naproxen at their IC50 values 180, 2.52, and 2.06 μg/mL, respectively. 2,4,5-TMBA, a natural inhibitor of cyclooxygenase-2, suppresses adipogenesis and oromotes lipolysis in 3T3-L1 adipocytes. 2,4,5-Trimethoxybenzaldehyde (2,4,5-TMBA) present in plant roots, seeds, and leaves is reported to be a significant inhibitor of cyclooxygenase-2 (COX-2) activity at the concentration of 100 μg/mL. Because COX-2 is associated with differentiation of preadipocytes, the murine 3T3-L1 cells are cultured with 100 μg/mL of 2,4,5-TMBA during differentiation and after the cells are fully differentiated to study the effect of 2,4,5-TMBA on adipogenesis and lipolysis. Oil Red O staining and triglyceride assay revealed that 2,4,5-TMBA inhibited the formation of lipid droplets during differentiation; moreover, 2,4,5-TMBA down-regulated the protein levels of adipogenic signaling molecules and transcription factors MAP kinase kinase (MEK), extracellular signal-regulated kinase (ERK), CCAAT/enhancer binding protein (C/EBP)α, β, and δ, peroxisome proliferator-activated receptor (PPAR)γ, adipocyte determination and differentiation-dependent factor 1 (ADD1), and the rate-limiting enzyme for lipid synthesis acetyl-CoA carboxylase (ACC). In fully differentiated adipocytes, treatment with 2,4,5-TMBA for 72 h significantly decreased lipid accumulation by increasing the hydrolysis of triglyceride through suppression of perilipin A (lipid droplet coating protein) and up-regulation of hormone-sensitive lipase (HSL). When treated with 100 μg/mL of 2,4,5-TMBA for 24, 48, or 72 h, the viability of fully differentiated 3T3-L1 adipocytes is decreased by 8.35, 15.54, and 27.26%, respectively. When the preadiocytes are treated with 100 μg/mL of 2,4,5-TMBA for 24 h before differentiation medium is supplemented, the cell viability is decreased by 26.46%[1]. A COX-2 inhibitor 2,4,5-trimethoxybenzaldehyde (TMBA) is found to be the most abundant constituent, but is totally absent in its cultured broth and its natural host, C. kanehirae wood. 2,4,5-trimethoxybenzaldehyde (TMBA) is the major constituent in fruiting bodies[2]. |
| Cell Assay | 3T3-L1 preadipocytes are seeded into 6-well plates at a concentration of 105/well and cultured in DMEM supplemented with 10% bovine calf serum at 37°C in a humidified atmosphere containing 5% CO2. Two days after confluence, cells are cultured in FBS-containing DMEM (10%, v/v) with the addition of adipogenic factors (0.5 mM IBMX, 1 μM DEX, 5 μg/mL insulin) to induce differentiation (Day 0). Two days later (Day 2), the medium is changed to DMEM supplemented with 10% FBS and 5 μg/mL insulin for another two days. Afterward (Day 4), the medium is changed to DMEM supplemented with 10% FBS only. For the coculture study, 2,4,5-TMBA (0.1 g dissolved in 2 mL of DMSO) is added to the medium from Day 0 to Day 8 (final concentration 100 μg/mL). Control samples are prepared by adding isovolumetric DMSO to the culture medium. For the postculture study, 2,4,5-TMBA is added to the medium on Day 8 (when the cells are fully differentiated) at a final concentration of 100 μg/mL, followed by another 72 h culture. 3T3-L1 cells are seeded in 96-well plates at a concentration of 104/well. Twenty-four hours after seeding, the cells are treated with 100 μg/mL of 2,4,5-TMBA for 24 h or for the whole 8-day differentiation period. Fully differentiated adipocytes are also treated with 100 μg/mL of 2,4,5-TMBA for 24-72 h to test the cytotoxicity. At the end of treatment, cells are cultured with MTT at a final concentration of 0.5 mg/mL for another 4 h. The purple MTT formazan is dissolved by DMSO and the absorbance at 570 nm is taken with a spectrophotometer. The absorbance is proportional to the viability of adipocytes[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 334.7±37.0 °C at 760 mmHg |
| Melting Point | 112-114 °C(lit.) |
| Molecular Formula | C10H12O4 |
| Molecular Weight | 196.200 |
| Flash Point | 149.0±26.5 °C |
| Exact Mass | 196.073563 |
| PSA | 44.76000 |
| LogP | 1.62 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.525 |
| Storage condition | 2-8°C |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CU8460000 |
| HS Code | 2912499000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2912499000 |
|---|---|
| Summary | 2912499000. other aldehyde-ethers, aldehyde-phenols and aldehydes with other oxygen function. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |





![1-[bis(2,4,5-trimethoxyphenyl)methyl]-2,4,5-trimethoxybenzene structure](https://www.chemsrc.com/caspic/355/14470-07-6.png)