meso-1,2-Diphenylethylenediamine
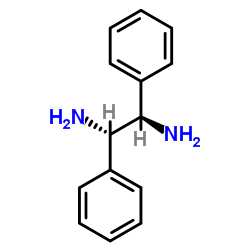
meso-1,2-Diphenylethylenediamine structure
|
Common Name | meso-1,2-Diphenylethylenediamine | ||
|---|---|---|---|---|
| CAS Number | 951-87-1 | Molecular Weight | 212.29000 | |
| Density | 1.106g/cm3 | Boiling Point | 353.9ºC at 760mmHg | |
| Molecular Formula | C14H16N2 | Melting Point | 118-122ºC | |
| MSDS | Chinese USA | Flash Point | 199.9ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1,2-diphenylethane-1,2-diamine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.106g/cm3 |
|---|---|
| Boiling Point | 353.9ºC at 760mmHg |
| Melting Point | 118-122ºC |
| Molecular Formula | C14H16N2 |
| Molecular Weight | 212.29000 |
| Flash Point | 199.9ºC |
| Exact Mass | 212.13100 |
| PSA | 52.04000 |
| LogP | 3.78700 |
| Index of Refraction | 1.619 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2921590090 |
| Precursor 10 | |
|---|---|
| DownStream 6 | |
| HS Code | 2921590090 |
|---|---|
| Summary | 2921590090. other aromatic polyamines and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Synthesis and characterisation of nickel Schiff base complexes containing the meso-1,2-diphenylethylenediamine moiety: selective interactions with a tetramolecular DNA quadruplex.
Dalton Trans. 44(7) , 3136-50, (2015) As part of a program of preparing metal complexes which exhibit unique affinities towards different DNA structures, we have synthesised the novel Schiff base complex N,N'-bis-4-(hydroxysalicylidine)me... |
|
|
Enantioseparation of 1-arylethanols via a supramolecular chiral host consisting of N-(2-naphthoyl)-L-aspartic acid and an achiral diamine.
Org. Biomol. Chem. 10(9) , 1877-82, (2012) A supramolecular chiral host consisting of N-(2-naphthoyl)-L-aspartic acid (L-1) and meso-1,2-diphenylethylenediamine (2) is effective in enantioseparation of 1-arylethanols (up to 96% ee with 100% in... |
|
|
Synthesis and antiviral activities of N-mono- and/or N,N'-di-carbamoyl and acyl derivatives of symmetrical diamines.
Chem. Pharm. Bull. 56(7) , 1052-8, (2008) N-carbamoyl and N-acyl diamine derivatives were synthesized from symmetrical diamines by their addition to iso(thio)cyanates, cleavage reaction of acid anhydride, or N-acylation by acyl chloride. (1R,... |
| (1R,2R)-(+)-1,2-Diphenyl-1,2-ethanediamine |
| meso-1,2-Diphenylethylenediamine |
| (1R,2R)-(+)-1,2-Diphenylethylenediamine |
| (1R,2S)-1,2-Diphenyl-1,2-ethanediamine |
| (1R,2R)-1,2-Diphenylethan-1,2-diamin |
| (1R,2R)-1,2-Diphenylethylenediamine |
| (1R,2R)-(+)-1,2-Diamino-1,2-diphenylethane |
| (1R,2S)-1,2-diphenylethane-1,2-diamine |
| CH06110 MESO-1,2-DIPHENYLETHYLENEDIAMINE |
| (1R,2R)-1,2-Diphenyl-1,2-ethanediamine |
| MESO-1 2-DIPHENYLETHYLENEDIAMINE |
| 1,2-ETHANEDIAMINE,1,2-DIPHENYL-, (1R,2S)-REL- |
| MFCD00274328 |
| 1,2-Ethanediamine, 1,2-diphenyl-, (1R,2S)- |
| (1R,2R)-(+)-1,2-Diphenyl-1,2-Ethanediamine ee |
| (1R,2R)-1,2-diphenylethane-1,2-diamine |
| 1,2-Ethanediamine, 1,2-diphenyl-, (1R,2R)- |
 CAS#:100-52-7
CAS#:100-52-7 CAS#:33577-29-6
CAS#:33577-29-6![N-[2-(benzylideneamino)-1,2-diphenylethyl]benzamide Structure](https://www.chemsrc.com/caspic/104/3190-22-5.png) CAS#:3190-22-5
CAS#:3190-22-5 CAS#:1666-17-7
CAS#:1666-17-7 CAS#:10229-53-5
CAS#:10229-53-5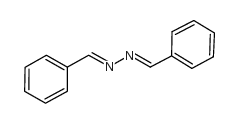 CAS#:28867-76-7
CAS#:28867-76-7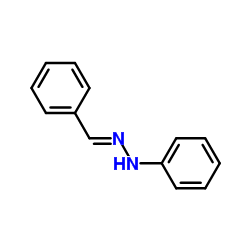 CAS#:588-64-7
CAS#:588-64-7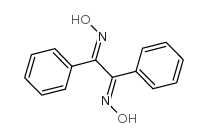 CAS#:23873-81-6
CAS#:23873-81-6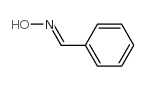 CAS#:932-90-1
CAS#:932-90-1![N-[(E)-2-nitroso-1,2-diphenyl-ethenyl]hydroxylamine Structure](https://www.chemsrc.com/caspic/447/572-45-2.png) CAS#:572-45-2
CAS#:572-45-2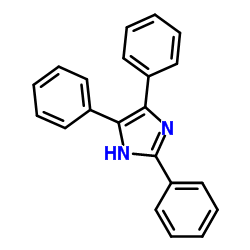 CAS#:484-47-9
CAS#:484-47-9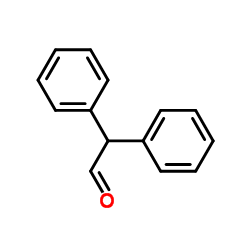 CAS#:947-91-1
CAS#:947-91-1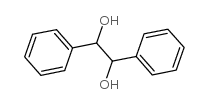 CAS#:655-48-1
CAS#:655-48-1 CAS#:29841-69-8
CAS#:29841-69-8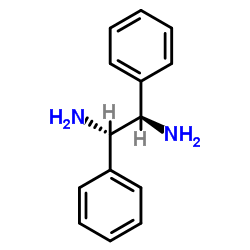 CAS#:35132-20-8
CAS#:35132-20-8 CAS#:642-04-6
CAS#:642-04-6