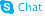CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
US7968700
-
CHEMICAL NAME :
-
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-4-(2,3-dichlorophenyl)-2,6-dimethyl-, ethyl methyl ester
-
CAS REGISTRY NUMBER :
-
72509-76-3
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C18-H19-Cl2-N-O4
-
MOLECULAR WEIGHT :
-
384.28
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1050 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
23 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5400 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 11,126,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
76 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
205 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3100 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 26,239,1995
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
200 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - respiratory depression Gastrointestinal - nausea or vomiting Blood - changes in spleen
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 20,2963,1992 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
13140 mg/kg/1Y-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - changes in ovarian weight
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 20,2999,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
4500 mg/kg/90D-I
-
TOXIC EFFECTS :
-
Related to Chronic Data - death
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 20,2963,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1674 mg/kg/1Y-I
-
TOXIC EFFECTS :
-
Musculoskeletal - changes in teeth and supporting structures
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 43,729,1993 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
22 mg/kg
-
SEX/DURATION :
-
male 43 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - breast development
-
REFERENCE :
-
MJAUAJ Medical Journal of Australia. (Australasian Medical Pub. Co. Ltd., 71-79 Arundel St., Glebe, N.S.W., Australia) V.1- 1914- Volume(issue)/page/year: 161,328,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
322 mg/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 20,3025,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2636 mg/kg
-
SEX/DURATION :
-
male 9 week(s) pre-mating female 2 week(s) pre-mating - 3 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 43,106,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
13834 ug/kg
-
SEX/DURATION :
-
female 14-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - maternal-fetal exchange
-
REFERENCE :
-
PHTOEH Pharmacology and Toxicology (Copenhagen). (Munksgaard International Pub., POB 2148, DK-1016 Copenhagen K, Denmark) V.60- 1987- Volume(issue)/page/year: 71,361,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
64400 ug/kg
-
SEX/DURATION :
-
female 6-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,3429,1994
|


![2-[(2,3-dichlorophenyl)methylene]-3-oxobutanoic acid ethyl ester structure](https://www.chemsrc.com/caspic/104/68064-64-2.png)


![ethyl 2-[(2,3-dichlorophenyl)methylidene]-3-oxobutanoate structure](https://www.chemsrc.com/caspic/157/94739-24-9.png)
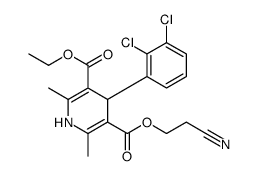
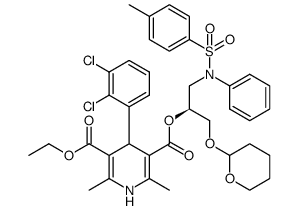
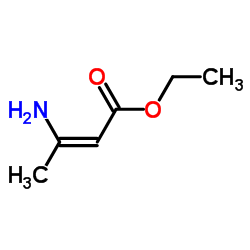
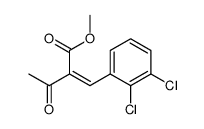
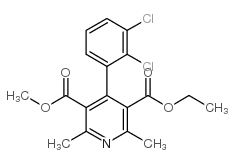 CAS#:96382-71-7
CAS#:96382-71-7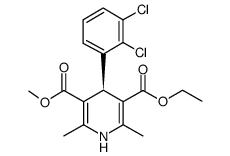 CAS#:119945-59-4
CAS#:119945-59-4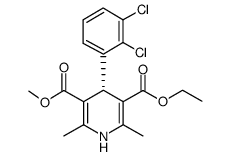 CAS#:105618-03-9
CAS#:105618-03-9