Zonisamide
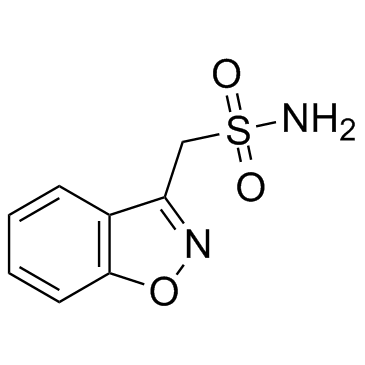
Zonisamide structure
|
Common Name | Zonisamide | ||
|---|---|---|---|---|
| CAS Number | 68291-97-4 | Molecular Weight | 212.226 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 457.2±47.0 °C at 760 mmHg | |
| Molecular Formula | C8H8N2O3S | Melting Point | 275°C dec. | |
| MSDS | N/A | Flash Point | 230.3±29.3 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of ZonisamideZonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug.Target: Calcium channel inhibitor; Sodium channel inhibitorZonisamide is a sulfonamide anticonvulsant approved for use as an adjunctive therapy in adults with partial-onset seizures for adults; infantile spasm, mixed seizure types of Lennox-Gastaut syndrome, myoclonic, and generalized tonic clonic seizure. Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug. It has shown activity in various animal models of epilepsy, and although a detailed mode of action awaits clarification it appears to block the propagation/spread of seizure discharges and to suppress the epileptogenic focus [1].Zonisamide 500 mg/day was significantly superior to placebo in reducing the frequency of complex partial seizures (-51% versus -16%), all partial seizures and all seizures, with dose-dependent benefit provided over a 100-500 mg/day dose range. Supporting trials have confirmed significant increases in reduction in median seizure frequency (up to 41%) and responder rates (35-42%) compared with placebo following zonisamide 400-600 mg/day, enabling 20-27% of patients to attain >or=75% reduction in seizure frequency [2].Clinical indications: Epilepsy; Lewy body dementia; Parkinsons diseaseToxicity: Anorexia; Somnolence; Dizziness; Irritability; Confusional state; Depression; Diplopia; Memory impairment |
| Name | zonisamide |
|---|---|
| Synonym | More Synonyms |
| Description | Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug.Target: Calcium channel inhibitor; Sodium channel inhibitorZonisamide is a sulfonamide anticonvulsant approved for use as an adjunctive therapy in adults with partial-onset seizures for adults; infantile spasm, mixed seizure types of Lennox-Gastaut syndrome, myoclonic, and generalized tonic clonic seizure. Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug. It has shown activity in various animal models of epilepsy, and although a detailed mode of action awaits clarification it appears to block the propagation/spread of seizure discharges and to suppress the epileptogenic focus [1].Zonisamide 500 mg/day was significantly superior to placebo in reducing the frequency of complex partial seizures (-51% versus -16%), all partial seizures and all seizures, with dose-dependent benefit provided over a 100-500 mg/day dose range. Supporting trials have confirmed significant increases in reduction in median seizure frequency (up to 41%) and responder rates (35-42%) compared with placebo following zonisamide 400-600 mg/day, enabling 20-27% of patients to attain >or=75% reduction in seizure frequency [2].Clinical indications: Epilepsy; Lewy body dementia; Parkinsons diseaseToxicity: Anorexia; Somnolence; Dizziness; Irritability; Confusional state; Depression; Diplopia; Memory impairment |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 457.2±47.0 °C at 760 mmHg |
| Melting Point | 275°C dec. |
| Molecular Formula | C8H8N2O3S |
| Molecular Weight | 212.226 |
| Flash Point | 230.3±29.3 °C |
| Exact Mass | 212.025558 |
| PSA | 94.57000 |
| LogP | -0.10 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Water Solubility | H2O: >5 mg/mL, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | 7-16-36/37-45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| HS Code | 2935009090 |
|
~89% 
Zonisamide CAS#:68291-97-4 |
| Literature: WO2007/16208 A2, ; Page/Page column 16-17 ; |
|
~75% 
Zonisamide CAS#:68291-97-4 |
| Literature: US2006/9644 A1, ; Page/Page column 10 ; |
|
~86% 
Zonisamide CAS#:68291-97-4 |
| Literature: US2004/14983 A1, ; Page 4-5 ; |
|
~% 
Zonisamide CAS#:68291-97-4 |
| Literature: US2007/66830 A1, ; Page/Page column 5 ; |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Application of surfactant assisted dispersive liquid-liquid microextraction as an efficient sample treatment technique for preconcentration and trace detection of zonisamide and carbamazepine in urine and plasma samples.
J. Chromatogr. A. 1308 , 25-31, (2013) A simple, rapid, and efficient method, based on surfactant assisted dispersive liquid-liquid microextraction (SA-DLLME), followed by high performance liquid chromatography (HPLC) has been developed fo... |
|
|
Zonisamide in clinical practice.
Acta Neurol. Scand.,. Suppl.c. (194) , 29-35, (2012) Zonisamide is currently licensed in Europe and the USA for the adjunctive treatment of partial seizures (with or without secondary generalization) in adults, based on the results of four pivotal, rand... |
|
|
Zonisamide in the treatment of bulimia nervosa: an open-label, pilot, prospective study.
Int. J. Eat. Disord. 46(7) , 747-50, (2013) To assess preliminarily the effectiveness of zonisamide in bulimia nervosa.This was an open-label, prospective, 12-week, flexible dose study of zonisamide in bulimia nervosa. The primary outcome was b... |
| Excegran |
| EINECS 200-659-6 |
| 3-sulfamoylmethyl-1,2-benzisoxazole |
| 1-(1,2-benzisoxazol-3-yl)méthanesulfonamide |
| 3-sulfamoyl-methyl-1,2-benzisoxazol |
| 1-(1,2-Benzisoxazol-3-yl)methansulfonamid |
| Zonisamide |
| 1-(1,2-benzisoxazol-3-yl)methanesulfonamide |
| ad-810 |
| Zonegran |
| exceglan |
| 1-(1,2-Benzoxazol-3-yl)methanesulfonamide |
| ZON |
| MFCD00865316 |
| Excemide |
| 1,2-Benzisoxazole-3-methanesulfonamide |
| excegram |
| ci-912 |

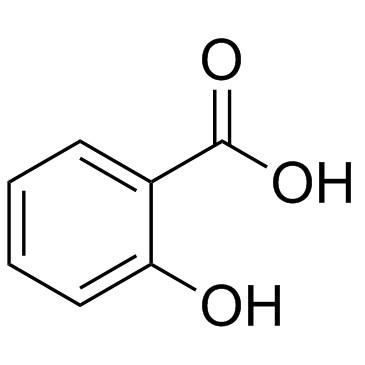 CAS#:69-72-7
CAS#:69-72-7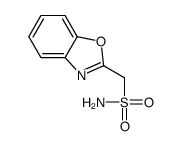 CAS#:73101-70-9
CAS#:73101-70-9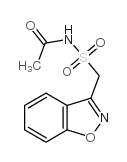 CAS#:68936-43-6
CAS#:68936-43-6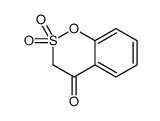 CAS#:49670-47-5
CAS#:49670-47-5