5-Nitro-1,10-phenanthroline
Modify Date: 2024-01-02 20:18:46
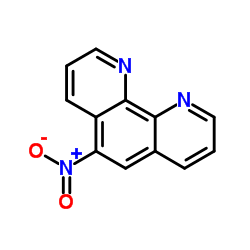
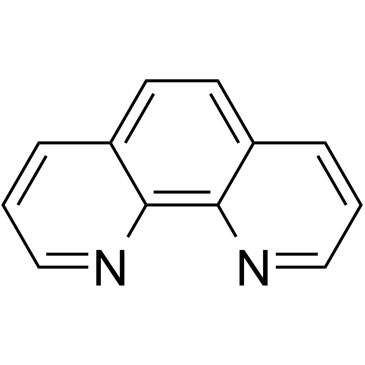
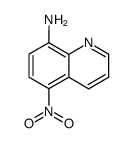
5-Nitro-1,10-phenanthroline structure
|
Common Name | 5-Nitro-1,10-phenanthroline | ||
|---|---|---|---|---|
| CAS Number | 4199-88-6 | Molecular Weight | 225.203 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 444.0±30.0 °C at 760 mmHg | |
| Molecular Formula | C12H7N3O2 | Melting Point | 202-204 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 222.3±24.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
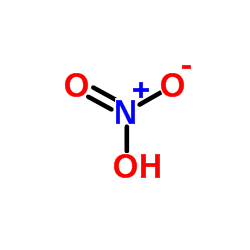
Use of 5-Nitro-1,10-phenanthroline5-Nitro-1,10-phenanthroline (5-NP), is a o-Phenanthroline (HY-W004544) derivative, as a mediator of glucose oxidase (GOX) with antituberculous activity. 5-Nitro-1,10-phenanthroline can be applied as redox mediators for oxidases and is suitable for the development of reagent-less biosensors and biofuel cells[1][1]. |
| Name | 5-Nitro-1,10-phenanthroline |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Nitro-1,10-phenanthroline (5-NP), is a o-Phenanthroline (HY-W004544) derivative, as a mediator of glucose oxidase (GOX) with antituberculous activity. 5-Nitro-1,10-phenanthroline can be applied as redox mediators for oxidases and is suitable for the development of reagent-less biosensors and biofuel cells[1][1]. |
|---|---|
| Related Catalog | |
| In Vitro | 5-Nitro-1,10-phenanthroline (25 μM; 24 h) kills naturally resistant intracellular bacteria by inducing autophagy in THP-1 macrophages[2]. 5-Nitro-1,10-phenanthroline (1x, 5x, 20x or 50x MIC, MIC=0.78 μM; 1 h) also modulates the host machinery to kill intracellular pathogens by inhibiting mycolic acid biosynthesis of Mtb[2]. Cell Viability Assay[2] Cell Line: Mtb H37Rv, M. bovis BCG and M. bovis BCG-5NP resistant strain Concentration: 0-12.5 μM Incubation Time: 24 hours Result: Inhibited pathogens with MIC99 values of 0.78 μM (Mtb H37Rv), 0.78 μM (M. bovis BCG), and >12.5 μM (M. bovis BCG-5NP), respectively. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 444.0±30.0 °C at 760 mmHg |
| Melting Point | 202-204 °C(lit.) |
| Molecular Formula | C12H7N3O2 |
| Molecular Weight | 225.203 |
| Flash Point | 222.3±24.6 °C |
| Exact Mass | 225.053833 |
| PSA | 71.60000 |
| LogP | 1.56 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.768 |
| Water Solubility | insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful;Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
|
~98% 
5-Nitro-1,10-ph... CAS#:4199-88-6 |
| Literature: Ji, Shaomin; Guo, Huimin; Yuan, Xiaolin; Li, Xiaohuan; Ding, Haidong; Gao, Peng; Zhao, Chunxia; Wu, Wenting; Wu, Wanhua; Zhao, Jianzhang Organic Letters, 2010 , vol. 12, # 12 p. 2876 - 2879 |
|
~42% 
5-Nitro-1,10-ph... CAS#:4199-88-6 |
| Literature: Nisshinbo Industries, Inc. Patent: US5856479 A1, 1999 ; |
|
~0% 
5-Nitro-1,10-ph... CAS#:4199-88-6 |
| Literature: Amouyal, Edmond; Homsi, Abdulrazzak; Chambron, Jean-Claude; Sauvage, Jean-Pierre Journal of the Chemical Society, Dalton Transactions: Inorganic Chemistry (1972-1999), 1990 , # 6 p. 1841 - 1845 |
|
~% 
5-Nitro-1,10-ph... CAS#:4199-88-6 |
| Literature: Halcrow; Kermack Journal of the Chemical Society, 1946 , p. 155 |
|
~% 
5-Nitro-1,10-ph... CAS#:4199-88-6 |
| Literature: Halcrow; Kermack Journal of the Chemical Society, 1946 , p. 155 |
|
~%
Detail
|
| Literature: Inglett; Smith Journal of the American Chemical Society, 1950 , vol. 72, p. 842 |
|
~%
Detail
|
| Literature: Smith; Cagle Journal of Organic Chemistry, 1947 , vol. 12, p. 781,782 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The effect of ancillary ligand chirality and phenanthroline functional group substitution on the cytotoxicity of platinum(II)-based metallointercalators.
J. Inorg. Biochem. 101 , 1049-1058, (2007) Fifteen platinum(II)-based metallointercalators have been synthesised that utilise substituted 1,10-phenanthroline (phen) ligands, including 5-chloro-1,10-phenanthroline (5-Cl-phen), 5-methyl-1,10-phe... |
| 5-nitro-1,10-phenenthroline |
| 1,10-Phenanthroline, 5-nitro- |
| 5-Nitro-1,10-phenanthroline |
| 5-Nitro-1,10-diazaphenanthrene |
| EINECS 224-097-6 |
| 5-nitro-1,10-phenantroline |
| 1,10-Phenanthroline,5-nitro |
| Nitroferroin |
| MFCD00004981 |