Vasopressin
Modify Date: 2024-01-05 17:25:05
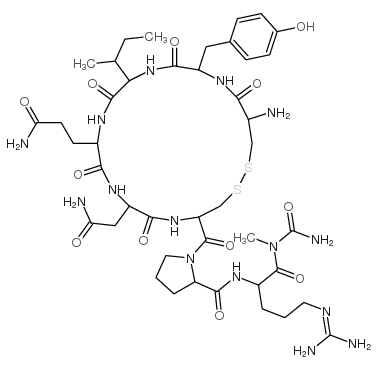
Vasopressin structure
|
Common Name | Vasopressin | ||
|---|---|---|---|---|
| CAS Number | 11000-17-2 | Molecular Weight | 1050.22000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C43H67N15O12S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of VasopressinVasopressin is a cyclic nonapeptide that is synthesized centrally in the hypothalamus. Vasopressin participates in the hypothalamic-pituitary-adrenal axis, and regulates pituitary corticotropin secretion by potentiating the stimulatory effects of corticotropin releasing factor. Vasopressin also can act as a neurotransmitter, exerting its action by binding to specific G protein-coupled receptors[1][2][3]. |
| Name | Vasopressin |
|---|---|
| Synonym | More Synonyms |
| Description | Vasopressin is a cyclic nonapeptide that is synthesized centrally in the hypothalamus. Vasopressin participates in the hypothalamic-pituitary-adrenal axis, and regulates pituitary corticotropin secretion by potentiating the stimulatory effects of corticotropin releasing factor. Vasopressin also can act as a neurotransmitter, exerting its action by binding to specific G protein-coupled receptors[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | AVP (0.01 nM-1 μM) induces Ca2+ increase in Chinese hamster ovary cells expressing rat or human V1b receptors[2]. |
| In Vivo | Vasopressin (0.03-0.3 μg/kg; i.p.) potentiates corticotropin release provoked by exogenous corticoliberin and increases corticotropin secretion subsequent to body water loss[2]. Vasopressin (0.001-0.1 mg/kg; i.p.) potently increases adjacent lying, where rats meeting for the first time lie passively next to each other[3]. |
| References |
| Molecular Formula | C43H67N15O12S2 |
|---|---|
| Molecular Weight | 1050.22000 |
| Exact Mass | 1049.45000 |
| PSA | 505.74000 |
| LogP | 1.50910 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| pituitrin |
| EINECS 200-050-5 |
| VASOPRESSIN ACETATE |
| MFCD03839092 |
| vasophysin |
| leiormone |
| tonephin |
| VASOPRASSIN |
| adh(hormone) |
| pitressin |