452-35-7
| Name | ethoxzolamide |
|---|---|
| Synonyms |
Ethamide
6-ethoxy-1,3-benzothiazole-2-sulfonamide Glaucotensil Ethoxyzolamide Ethoxazolamide 2-Benzothiazolesulfonamide,6-ethoxy EINECS 207-199-5 MFCD00057089 Etoxzolamide 6-Ethoxy-2-benzothiazolesulfonamide Cardrase Diuretic C 6-ethoxybenzothiazole-2-sulphonamide |
| Description | Ethoxzolamide is a carbonic anhydrase inhibitor with Ki of 1 nM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 1 nM (carbonic anhydrase)[1] |
| In Vitro | Ethoxzolamide (ETZ) treatment causes >90% inhibition of reporter GFP fluorescence in infected macrophages. Moreover, in a 9-day macrophage survival assay, Ethoxzolamide (ETZ) treatment significantly inhibits the ability of M. tuberculosis to grow intracellularly[2]. |
| In Vivo | It is discovered that the lipid-soluble ethoxzolamide is converted in vivo to a water-soluble metabolite, while retaining high activity againstt the enzyme. At the minimal dose for maximal effect (4 mg/kg i.v. at 45 min) the IOP lowering is 4.2 mmHg, the concentration in anterior uvea is 2.5 pmol/kg, and the fractional inhibition of the enzyme (i) is 0.9995. The effect declines rapidly, attributable to the very short half-life of drug in plasma, leading to depletion of free drug in the anterior uvea and other tissues[1]. Ethoxzolamide (ETZ) strongly downregulates GFP reporter fluorescence in mouse lungs, with 3-fold inhibition of GFP signal compare to that in the mock-treating control. There is a significant reduction of bacterial survival in the lungs of ETZ-treating mice compare to the mock-treating control[2]. |
| Cell Assay | BMDMs are treated with 80 μM Ethoxzolamide (ETZ) or an equivalent volume of DMSO every 2 days for 9 days total. At days 3, 6, and 9, intracellular bacteria are quantified by lysing macrophage monolayers and performing serial dilution plating of lysates on 7H10 agar. For fluorescence microscopy experiments, macrophages are seeded on glass coverslips before infection with M. tuberculosis CDC1551. Samples are treated every 2 days with 100 μM Ethoxzolamide (ETZ) or an equal volume of DMSO for 9 days[2]. |
| Animal Admin | Rats (male, 300–325 g) are randomly selected into 2 groups (n=6 each group) and Ethoxzolamide (EZ) is administered at a dose of 0.18 mg/kg (in PEG 300: ethanol, 1:1) via i.v. injection through the tail vein. Blood samples (about 50-100 µL) are collected in heparinizing tubes at 0, 15, 30, 60, 120, 180, 240, 360, 540, and 1440 min post-injection, via tail snip with isoflurane as anesthetic. Plasma samples are prepared and stored at -80 °C until analysis. To study the distribution in brain, rats in group 1 are scarified at 6 hours and rats in group 2 are scarified at 24 hours to collect the brain tissues. Those blood samples from group 2 are analyzed to generated PK profile[3]. |
| References |
| Density | 1.47g/cm3 |
|---|---|
| Boiling Point | 464.9ºC at 760mmHg |
| Melting Point | 190-193ºC(lit.) |
| Molecular Formula | C9H10N2O3S2 |
| Molecular Weight | 258.31700 |
| Flash Point | 235ºC |
| Exact Mass | 258.01300 |
| PSA | 118.90000 |
| LogP | 3.12350 |
| Vapour Pressure | 8.05E-09mmHg at 25°C |
| Index of Refraction | 1.644 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DL6390000 |
| HS Code | 2935009090 |
|
~87% 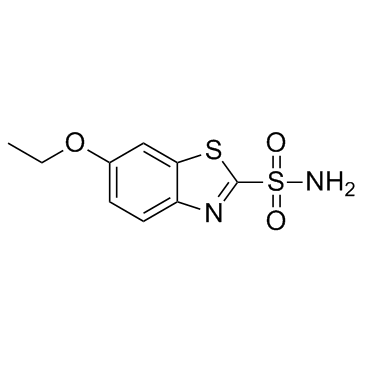
452-35-7 |
| Literature: Larsen, R.D.; Roberts, F.E. Synthetic Communications, 1986 , vol. 16, # 8 p. 899 - 904 |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |

![N-[(6-ethoxy-1,3-benzothiazol-2-yl)sulfonyl]acetamide structure](https://www.chemsrc.com/caspic/051/88946-18-3.png)
![6-ETHOXYBENZO[D]THIAZOLE-2-CARBONITRILE structure](https://www.chemsrc.com/caspic/407/91634-13-8.png)