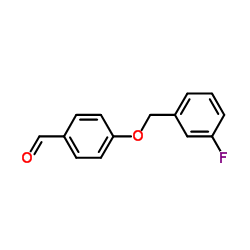
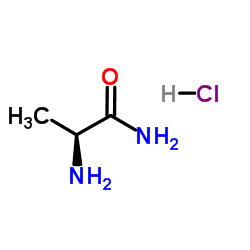
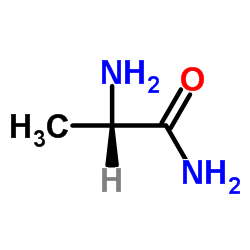
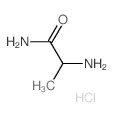
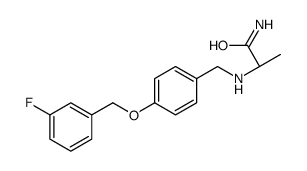
133865-89-1
| Name | (2S)-2-[[4-[(3-fluorophenyl)methoxy]phenyl]methylamino]propanamide |
|---|---|
| Synonyms |
(S)-(+)-2-[4-(3-fluorobenzyloxy)benzylamino]-propanamide
(2S)-2-[4-(3-fluorobenzyloxy)benzylamino]propionamide Safinamide N-{4-[(3-Fluorobenzyl)oxy]benzyl}-L-alaninamide (2S)-2-[[[4-[(3-Fluorophenyl)methoxy]phenyl]methyl]amino]propanamide (S)-2-[[4-[(3-Fluorobenzyl)oxy]benzyl]amino]propionamide ZVY1&M1R DO1R CF &&L or S Form Propanamide, 2-[[[4-[(3-fluorophenyl)methoxy]phenyl]methyl]amino]-, (2S)- |
| Description | Safinamide (EMD 1195686; FCE 26743) selectively and reversibly inhibits MAO-B with IC50 of 98 nM, exhibits 5918-fold selectivity against MAO-A.IC50 value: 98 nM [1]Target: MAO-BSafinamide (EMD 1195686; FCE 26743; ) is a highly selective and reversible monoamine oxidase type B (MAO-B) inhibitor that increases neostriatal dopamine concentration. In addition, Safinamide (EMD 1195686; FCE 26743; ) is voltage-dependent sodium and calcium channel blocker. Safinamide (EMD 1195686; FCE 26743; ) appears to bind to the batrachotoxin-sensitive site 2 of the voltage-sensitive sodium channels. Safinamide blocks N and L-type calcium channels and inhibits glutamate and aspartate release from synaptic terminals. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 476.7±40.0 °C at 760 mmHg |
| Melting Point | 208-212° |
| Molecular Formula | C17H19FN2O2 |
| Molecular Weight | 302.343 |
| Flash Point | 242.1±27.3 °C |
| Exact Mass | 302.143066 |
| PSA | 64.35000 |
| LogP | 2.37 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.570 |
| Hazard Codes | Xi |
|---|---|
| RTECS | TX1457385 |
|
~87% 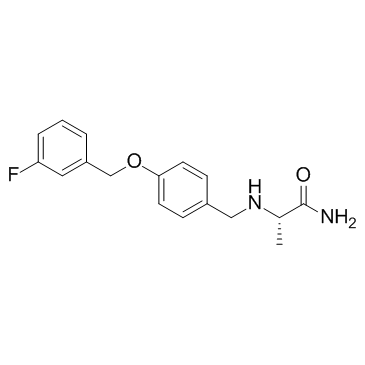
133865-89-1 |
| Literature: NEWRON PHARMACEUTICALS S.P.A. Patent: WO2009/74478 A1, 2009 ; Location in patent: Page/Page column 60-62 ; |
|
~92% 
133865-89-1 |
| Literature: NEWRON PHARMACEUTICALS S.P.A. Patent: WO2007/147491 A1, 2007 ; Location in patent: Page/Page column 34-35 ; |
|
~89% 
133865-89-1 |
| Literature: NEWRON PHARMACEUTICALS S.P.A. Patent: WO2007/147491 A1, 2007 ; Location in patent: Page/Page column 32 ; |
|
~% 
133865-89-1 |
| Literature: WO2009/74478 A1, ; Page/Page column 71-72 ; |
|
~% 
133865-89-1 |
| Literature: WO2009/74478 A1, ; Page/Page column 64-65 ; |
|
~% 
133865-89-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 50, # 20 p. 4909 - 4916 |