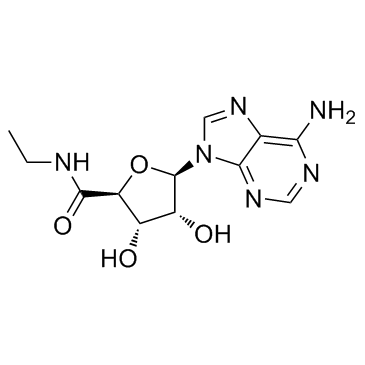
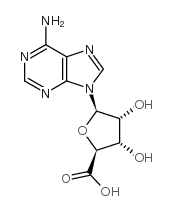
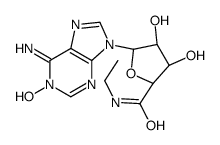
35920-39-9
| Name | N-ethyl-5'-carboxamidoadenosine |
|---|---|
| Synonyms |
(2S,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (non-preferred name)
(2S,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydro-2-furancarboxamide (non-preferred name) 5’-Ethylcarboxamido Adenosine MFCD00069195 5'-Ethylcarboxamido Adenosine 1-(6-AMINO-9H-PURIN-9-YL)-1-DEOXY-N-ETHYL-β-D-RIBOFURANURONAMIDE β-D-Ribofuranuronamide, 1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl- 5'-(N-Ethylcarboxamido)adenosine NECA |
| Description | 5'-N-Ethylcarboxamidoadenosine (NECA) is a nonselective adenosine receptor agonist. |
|---|---|
| Related Catalog | |
| Target |
Adenosine receptor[1] |
| In Vivo | After the administration of 5'-N-Ethylcarboxamidoadenosine (NECA), the mean number of cocaine infusions obtained per session is decreased significantly in a dose-dependent manner [5'-N-Ethylcarboxamidoadenosine (NECA): F(4,12)=14.9; P<0.001]. The administration of 5'-N-Ethylcarboxamidoadenosine (NECA) [F(4,12)=16.1; P<0.001] results in a significant increase in latencies above values obtained for vehicle treatment[1]. Daily i.p. injection of 5'-N-Ethylcarboxamidoadenosine (NECA) at 0.3 mg/kg/day for two weeks reduces malondialdehyde (MDA) levels in diabetic rats, but does not affect control rats. Daily treatment with NECA (0.3 mg/kg/day, i.p. for two weeks) reduces diabetes-induced gene expression of tumor necrosis factor (TNF)-α and interleukin (IL)-18 in diabetic rats, but does not affect control rats. Daily i.p. injection of 5'-N-Ethylcarboxamidoadenosine (NECA) at 0.3 mg/kg/day for two weeks also blocks the activation of JNK MAPK in diabetic rats, but does not affect control rats[2]. |
| Animal Admin | Male Wistar rats are used in this experiment. These animals range in weight between 350 and 425 g. They are housed individually in hanging wire cages under a 12-h light/dark cycle. The effects of 5'-N-Ethylcarboxamidoadenosine (NECA) are tested in four rats. In the experiment, either 5'-N-Ethylcarboxamidoadenosine (NECA) (5, 7.5, 10, 20 μg/kg) or vehicle (saline) is administered intraperitoneally 15 min prior to the start of test sessions. 5'-N-Ethylcarboxamidoadenosine (NECA) is administered following a random order crossover design. In most cases, animals are tested twice with the same dose[1]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Melting Point | 229-231ºC |
| Molecular Formula | C12H16N6O4 |
| Molecular Weight | 308.293 |
| Exact Mass | 308.123291 |
| PSA | 148.41000 |
| LogP | -0.48 |
| Index of Refraction | 1.829 |
| Water Solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.2 mg/mL Solutions may be stored for several days at 4?#x00b0;C. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic; |
| Risk Phrases | R28 |
| Safety Phrases | 22-26-36-45 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | VJ2232000 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| HS Code | 2934999090 |
| Precursor 7 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |