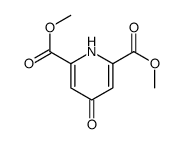
138-60-3
| Name | Chelidamic acid |
|---|---|
| Synonyms |
Pd(OH)2/C
chelidamic acid 4-Hydroxypyridine-2,6-dicarboxylic Acid Monohydrate 4-oxo-1,4-dihydro-2,6-pyridinedicarboxylic acid MFCD00066478 4-Hydroxy-2,6-pyridinedicarboxylic acid 4-oxo-1,4-dihydropyridine-2,6-dicarboxylic acid 4-PYRIDONE-2,6-DICARBOXYLIC ACID 1,4-dihydro-4-oxo-2,6-pyridinedicarboxylic acid .HELIDAMIC AICD 2,6-Pyridinedicarboxylic acid, 1,4-dihydro-4-oxo- CHELIDAMIC ACID HYDRATE Chelidamaic acid Chelidamic Acid Monohydrate Chelidamic aicd 2,6-Pyridinedicarboxylic acid, 4-hydroxy- Ammox-chelidonic acid 4-Hydroxypyridine-2,6-dicarboxylic acid chelidamic acid approx. EINECS 205-335-8 4-hydroxyl-pyridine-2,6-dicarboxylic acid |
| Description | Chelidamic acid is a heterocyclic organic acid with a pyran skeleton. Chelidamic acid has good coordination ability with noble metal ions. Chelidamic acid is also one of the most potent inhibitors of glutamate decarboxylase, with a Ki of 33 μM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 33 μM (Glutamate decarboxylase)[3]. |
| In Vitro | Chelidamic acid is a heterocyclic organic acid with a pyran skeleton[1]. Chelidamic acid has good coordination ability with noble metal ions[2]. Chelidamic acid is also one of the most potent inhibitors of glutamate decarboxylase, with a Ki of 33 μM[3]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 428.3±45.0 °C at 760 mmHg |
| Melting Point | 267 °C (dec.)(lit.) |
| Molecular Formula | C7H5NO5 |
| Molecular Weight | 183.118 |
| Flash Point | 212.8±28.7 °C |
| Exact Mass | 183.016769 |
| PSA | 107.46000 |
| LogP | -1.43 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.635 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933399090 |
|
~92% 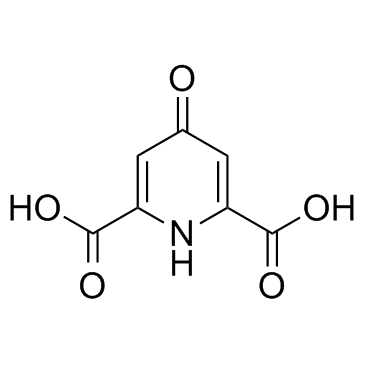
138-60-3 |
| Literature: Horvath, Gyoergy; Rusa, Cristian; Koentoes, Zoltan; Gerencser, Janos; Huszthy, Peter Synthetic Communications, 1999 , vol. 29, # 21 p. 3719 - 3731 |
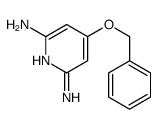
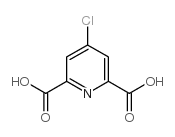
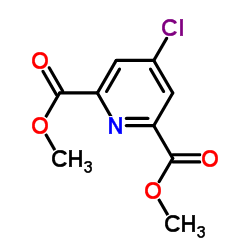
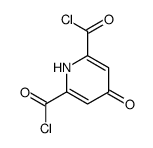
| Precursor 1 | |
|---|---|
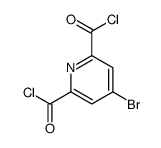
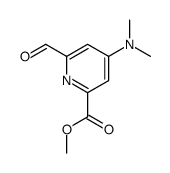
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![[4-chloro-6-(hydroxymethyl)pyridin-2-yl]methanol structure](https://www.chemsrc.com/caspic/470/1817-20-5.png)