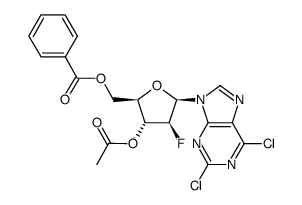
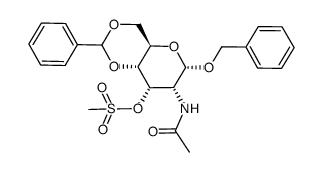
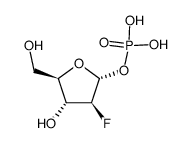
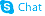123318-82-1
| Name | clofarabine |
|---|---|
| Synonyms |
Clofarex
(2R,3R,4S,5R)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol (2R,3R,4S,5R)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-2-(hydroxyméthyl)tétrahydrofuran-3-ol 9H-Purin-6-amine, 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)- 2-Chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)adenine (2R,3R,4S,5R)-5-(6-Amino-2-chlor-9H-purin-9-yl)-4-fluor-2-(hydroxymethyl)tetrahydrofuran-3-ol (2R,3R,4S,5R)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol 2-chloro-2'-arabino-fluoro-2'-deoxyadenosine Cl-F-Ara-A Clofarabine Clofarabine [USAN] Clolar 2-Chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-9H-purin-6-amine MFCD00871077 |
| Description | Clofarabine(Clolar; Clofarex) inhibits the enzymatic activities of ribonucleotide reductase (IC50 = 65 nM) and DNA polymerase.IC50 Value: 65 nMTarget: in vitro: Clofarabine is a second generation purine nucleoside analog with antineoplastic activity. It is phosphorylated intracellularly, which inhibits the enzymatic activities of ribonucleotide reductase (IC50 = 65 nM) and DNA polymerase, resulting in inhibition of DNA repair and synthesis of DNA and RNA. This nucleoside analog also disrupts mitochondrial function and membrane integrity, resulting in the release of pre-apoptotic factors, including cytochrome C and apoptotic-inducing factor, which activate apoptosis.in vivo: Clofarabine is used for treating relapsed or refractory acute lymphoblastic leukaemia (ALL) in children, after at least two other types of treatment have failed. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 599.5±60.0 °C at 760 mmHg |
| Melting Point | 228-2310C |
| Molecular Formula | C10H11ClFN5O3 |
| Molecular Weight | 303.677 |
| Flash Point | 316.4±32.9 °C |
| Exact Mass | 303.053436 |
| PSA | 119.31000 |
| LogP | 0.24 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.844 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | Xi,C |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . |
| Safety Phrases | S26-S36-S45-S36/37/39 |
| RIDADR | UN 2735 8/PG 3 |
| WGK Germany | 3 |
| RTECS | DP5550000 |
| Packaging Group | III |
| Hazard Class | 8 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |