CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
MJ8810000
-
CHEMICAL NAME :
-
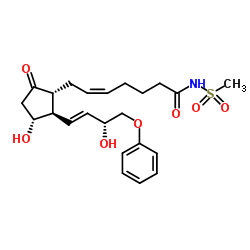
5-Heptenamide, 7-(3-hydroxy-2-(3-hydroxy-4-phenoxy-1-butenyl)-5-oxoc yclopentyl)- N-(methylsulfonyl)-, (1R-(1-alpha(Z),2-beta(1E,3R*),3-alpha))-
-
CAS REGISTRY NUMBER :
-
60325-46-4
-
LAST UPDATED :
-
199803
-
DATA ITEMS CITED :
-
14
-
MOLECULAR FORMULA :
-
C23-H31-N-O7-S
-
MOLECULAR WEIGHT :
-
465.61
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EKFAE9 Eksperimental'naya i Klinicheskaya Farmakologiya. Experimental and Clinical Pharmacology. (Mezhdunarodnaya Kniga, ul. B. Yakimanka, 39, 117049 Moscow, Russia) V.55- 1992- Volume(issue)/page/year: 56(4),37,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
18 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EKFAE9 Eksperimental'naya i Klinicheskaya Farmakologiya. Experimental and Clinical Pharmacology. (Mezhdunarodnaya Kniga, ul. B. Yakimanka, 39, 117049 Moscow, Russia) V.55- 1992- Volume(issue)/page/year: 56(4),37,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
3 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EKFAE9 Eksperimental'naya i Klinicheskaya Farmakologiya. Experimental and Clinical Pharmacology. (Mezhdunarodnaya Kniga, ul. B. Yakimanka, 39, 117049 Moscow, Russia) V.55- 1992- Volume(issue)/page/year: 56(4),37,1993 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
17 ug/kg
-
SEX/DURATION :
-
female 23 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
AJOGAH American Journal of Obstetrics and Gynecology. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1920- Volume(issue)/page/year: 137,8,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
10 ug/kg
-
SEX/DURATION :
-
female 9 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 19,29,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
20 ug/kg
-
SEX/DURATION :
-
female 49 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 22,471,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
30 ug/kg
-
SEX/DURATION :
-
female 15 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 26,279,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
15 ug/kg
-
SEX/DURATION :
-
female 7 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - abortion
-
REFERENCE :
-
GOBIDS Gynecologic and Obstetric Investigation. (S. Karger Pub., Inc., 79 Fifth Ave., New York, NY 10003) V.9- 1978- Volume(issue)/page/year: 29,10,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
5 ug/kg
-
SEX/DURATION :
-
female 10 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 27,51,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraplacental
-
DOSE :
-
500 ng/kg
-
SEX/DURATION :
-
female 15 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 26,279,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intracervical
-
DOSE :
-
1 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 16,377,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 6-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
EKFAE9 Eksperimental'naya i Klinicheskaya Farmakologiya. Experimental and Clinical Pharmacology. (Mezhdunarodnaya Kniga, ul. B. Yakimanka, 39, 117049 Moscow, Russia) V.55- 1992- Volume(issue)/page/year: 56(4),37,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 ug/kg
-
SEX/DURATION :
-
female 21-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
44LQAF "International Sulprostone Symposium, Papers, Vienna, 1978," Friebel, K., et al., eds., Berlin, Fed. Rep. Ger., Schering AG, 1979 Volume(issue)/page/year: -,39,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
120 ug/kg
-
SEX/DURATION :
-
female 63-64 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
ACEDAB Acta Endocrinologica, Supplementum (Copenhagen). (Periodica, Skolegade 12 E, DK-2500 Valby, Denmark) No.1- 1948- Volume(issue)/page/year: 253,148,1983
|



![7α-hydroxy-6β-(3'-hydroxy-4'-phenoxy-1'E-butenyl)-2-oxabicyclo[3.3.0]octan-3-one structure](https://www.chemsrc.com/caspic/359/51638-93-8.png)
![(3aR,4R,5R,6aS)-4-((3R,E)-4-phenoxy-3-((tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-5-((tetrahydro-2H-pyran-2-yl)oxy)hexahydro-2H-cyclopenta[b]furan-2-ol structure](https://www.chemsrc.com/caspic/169/286840-19-5.png)
![(3aR,4R,5R,6aS)-Hexahydro-4-[(E)-(3R)-4-phenoxy-3-[(tetrahydro-2H-pyran-2-yl)oxy]-1-butenyl]-5-[(tetrahydro-2H-pyran-2-yl)oxy]-2H-cyclopenta[b]furan-2-one structure](https://www.chemsrc.com/caspic/353/54347-99-8.png)
![(1S*,5R*,6R*,7R*)-7-trimethylsilyloxy-3-oxo-2-oxabicyclo[3.3.0]octane-6-carbaldehyde structure](https://www.chemsrc.com/caspic/167/122517-79-7.png)