264218-23-7
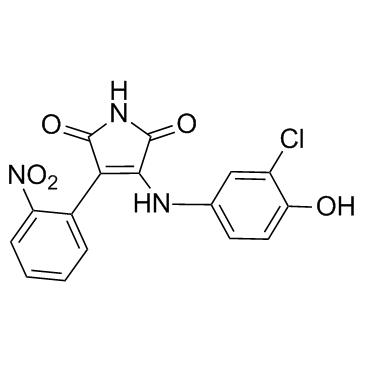
| Name | 3-(3-chloro-4-hydroxyanilino)-4-(2-nitrophenyl)pyrrole-2,5-dione |
|---|---|
| Synonyms |
1H-Pyrrole-2,5-dione, 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-
3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione Tocris-1617 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione 3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione MFCD04039789 SB 415286 |
| Description | SB 415286 is a potent and selective cell permeable inhibitor of GSK-3α, with an IC50 of 77.5 nM, and a Ki of 30.75 nM; SB 415286 is equally effective at inhibiting human GSK-3α and GSK-3β. |
|---|---|
| Related Catalog | |
| Target |
hGSK-3α:77.5 nM (IC50) hGSK-3β:77.5 nM (IC50) |
| In Vitro | SB 415286 (SB-415286) inhibits human GSK-3α with an IC50 of 77.5 nM, and a Ki of 30.75 nM. SB-415286 stimulates glycogen synthesis in the Chang human liver cell line with EC50 of 2.9 μM. SB-415286 stimulates glycogen synthase activity in Chang human liver cells. SB-415286 induces transcription of a β-catenin-LEF/TCF regulated reporter gene in HEK293 cells[1]. SB 415286 (SB-415286, 5-44 μM) attenuates B65 cell loss mediated by 1 mM H2O2. SB-415286 (5-44 μM) causes a significant dose-dependent decrease in the fluorescence intensity of DCF, and attenuates B65 ROS production as mediated by 1 mM H2O2. SB-415286 (5-44 μM) also attenuates ROS production in CGN mediated by 1 mM H2O2[2]. SB-415286 (50 µM) induces a substantial suppression of immunoprecipitated GSK3 activity by 97%[3]. |
| Kinase Assay | GSK-3 kinase activity is measured, in the presence or absence of SB-216763 or SB-415286, in a reaction mixture containing final concentrations of: 1 nM human GSK-3α or rabbit GSK3α; 50 mM MOPS pH 7.0; 0.2 mM EDTA; 10 mM Mg-acetate; 7.5 mM β-mercaptoethanol; 5% (w/v) glycerol; 0.01% (w/v) Tween-20; 10% (v/v) DMSO; 28 μM GS-2 peptide substrate. The GS-2 peptide sequence corresponds to a region of glycogen synthase that is phosphorylated by GSK-3. The assay is initiated by the addition of 0.34 μCi [33P]γ-ATP (IC50 determinations) or 2.7 μCi [33P]γ-ATP (Ki determinations). The total ATP concentration is 10 μM (IC50 determinations) or ranges from 0 to 45 μM (Ki determinations). Following 30 min incubation at room temperature the assay is stopped by the addition of one third assay volume of 2.5% (v/v) H3PO4 containing 21 mM ATP. Samples are spotted onto P30 phosphocellulose mats and these are washed six times in 0.5% (v/v) H3PO4. The filter mats are sealed into sample bags containing Wallac betaplate scintillation fluid. 33P incorporation into the substrate peptide is determined by counting the mats in a Wallac microbeta scintillation counter[1]. |
| Cell Assay | B65 cells are used after 24 h of in vitro culture. CGN are used after 7-8 days in vitro. Lithium and SB-415286 are dissolved in culture media and DMSO, respectively, and added to the neuronal preparation at the precise concentrations, 1 h before addition H2O2 (50 μM to 1 mM). To assess the loss in cell viability, we use the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium] method. MTT is added to the cells at a final concentration of 250 μM and incubated for 1 h, allowing the reduction in MTT to produce a dark blue formazan product. Media are then removed, and cells are dissolved in dimethylsulfoxide. Formazan production is measured by the absorbency change at 595 nm using a microplate reader. Viability results are expressed as percentages. The absorbency measured from non-treated cells is taken to be 100%[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 595.8±50.0 °C at 760 mmHg |
| Molecular Formula | C16H10ClN3O5 |
| Molecular Weight | 359.721 |
| Flash Point | 314.1±30.1 °C |
| Exact Mass | 359.030884 |
| PSA | 124.25000 |
| LogP | 2.06 |
| Appearance | yellow to orange |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.746 |
| Storage condition | −20°C |
| Water Solubility | DMSO: 16 mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |