554-57-4
| Name | Methazolamide |
|---|---|
| Synonyms |
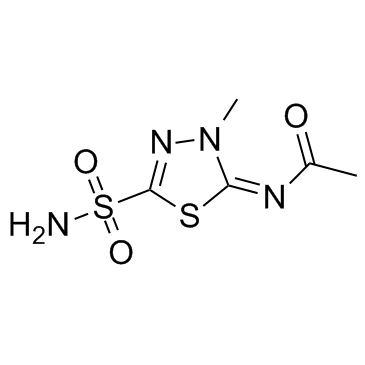
N-[(2E)-5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]acetamide
methazolamide Methazolamide [BAN:INN:JAN] Acetamide, N-[(2E)-5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]- Methazolamide (JAN/USP) EINECS 209-066-7 N-(4-Methyl-2-sulfamoyl-D2-1,3,4-thiadiazolin-5-ylidene)acetamide Metazolamide [DCIT] Acetamide, N-[(2Z)-5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]- 5-Acetylamino-4-methyl-D2-1,3,4-thiadiazoline-2-sulfonamide NEPTAZANE N1-[5-(aminosulfonyl)-3-methyl-2,3-dihydro-1,3,4-thiadiazol-2-yliden]acetamide 4-27-00-08221 (Beilstein Handbook Reference) N-[(2Z)-3-Methyl-5-sulfamoyl-1,3,4-thiadiazol-2(3H)-ylidene]acetamide N-[5-(Aminosulfonyl)-3-methyl-1,3,4-triadiazol-2(3H)-ylidene]acetamide N-[(2E)-3-Methyl-5-sulfamoyl-1,3,4-thiadiazol-2(3H)-ylidene]acetamide MFCD00083416 N-(3-methyl-5-sulfamoyl-1,3,4-thiadiazol-2-ylidene)acetamide |
| Description | Methazolamide is a carbonic anhydrase inhibitor used to treat glaucoma.Target: Carbonic AnhydraseMethazolamide is a carbonic anhydrase inhibitor with Ki of 50 nM, 14 nM and 36 nM for hCA I, hCA II and bCA IV isoforms, respectively [1]. Methazolamide is of strength equal to acetazolamide, another carbonic anhydrase inhibitor used to treat irregular breathing disorders. However, methazolamide differs from acetazolamide in that it fails to activate Ca2+-dependent potassium channels in skeletal muscles. Methazolamide does not impair respiratory work performance in anesthetized rabbits [2]. Oral administration of methazolamide decreases IOPs and AHFRs in clinically normal dogs, with effectiveness diminishing in the evening [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 402.0±28.0 °C at 760 mmHg |
| Melting Point | 208ºC (dec.) |
| Molecular Formula | C5H8N4O3S2 |
| Molecular Weight | 236.272 |
| Flash Point | 196.9±24.0 °C |
| Exact Mass | 236.003784 |
| PSA | 144.03000 |
| LogP | 0.13 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.737 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H351 |
| Precautionary Statements | P261-P280-P301 + P312 + P330 |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R40 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AC6350000 |
| HS Code | 2935009090 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
![5-acetylimino-4-methyl-4,5-dihydro-[1,3,4]thiadiazole-2-sulfonyl chloride structure](https://www.chemsrc.com/caspic/094/114224-34-9.png)