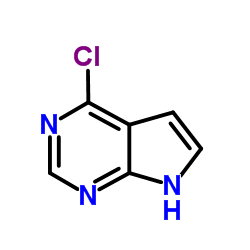
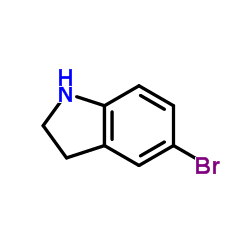
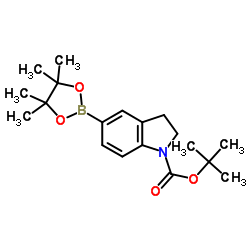
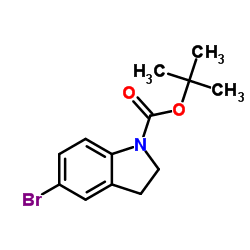
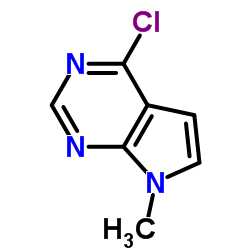
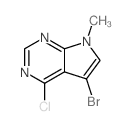
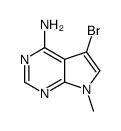
1337531-36-8
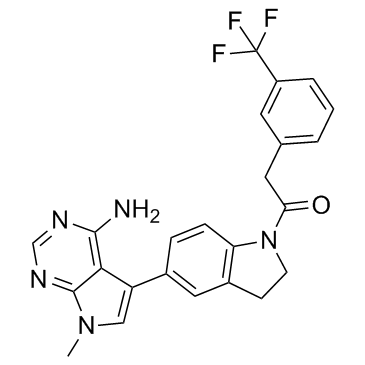
| Name | 1-[5-(4-amino-7-methylpyrrolo[2,3-d]pyrimidin-5-yl)-2,3-dihydroindol-1-yl]-2-[3-(trifluoromethyl)phenyl]ethanone |
|---|---|
| Synonyms |
CS-1428
QC-9698 1-[5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-2,3-dihydro-1H-indol-1-yl]-2-[3-(trifluoromethyl)phenyl]ethanone Ethanone, 1-[5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-2,3-dihydro-1H-indol-1-yl]-2-[3-(trifluoromethyl)phenyl]- 7-methyl-5-(1-([3-(trifluoromethyl)phenyl]acetyl)-2,3-dihydro-1H-indol-5-yI)-7H-pyrrolo[2,3-d]pyrimidin-4-amine S7307 GSK2606414 GSK-2606414 |
| Description | GSK2606414 is a cell-permeable and orally available PERK inhibitor with an IC50 of 0.4 nM. |
|---|---|
| Related Catalog | |
| Target |
EIF2AK3 (PERK):0.4 nM (IC50) EIF2AK1 (HRI):420 nM (IC50) EIF2AK2 (PKR):696 nM (IC50) |
| In Vitro | GSK2606414 inhibits PERK activation in cells[1]. |
| In Vivo | GSK2606414 (50 and 150 mg/kg, p.o.) inhibits the growth of a human tumor xenograft in mice[1]. |
| Animal Admin | Exponentially growing BxPC3 tumor cells (10×106 cells/mouse) from cell culture are implanted subcutaneously into the right flank of female nude mice. Sixteen days after implantation, mice with -200 mm3 tumors are randomized into various treatment groups (n=8 mice/group). Animals are orally treated with vehicle (0.5% hydoxypropylmethylcellulose, 0.1% Tween 80 in water, pH 4.8), compound at 50 or 150 mg/kg, b.i.d. for 21 days. Tumor volume is measured twice weekly with calipers and calculated. Results are represented as percent inhibition on completion of dosing, which is 100[1-(average growth of drug-treated population)/(average growth of vehicle-treated control population)]. Statistical analysis is performed using a two-tailed t test. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 720.3±60.0 °C at 760 mmHg |
| Molecular Formula | C24H20F3N5O |
| Molecular Weight | 451.444 |
| Flash Point | 389.4±32.9 °C |
| Exact Mass | 451.161987 |
| PSA | 77.04000 |
| LogP | 4.53 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | -20°C |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
|
~% 
1337531-36-8 |
| Literature: Axten, Jeffrey M.; Medina, Jesus R.; Feng, Yanhong; Shu, Arthur; Romeril, Stuart P.; Grant, Seth W.; Li, William Hoi Hong; Heerding, Dirk A.; Minthorn, Elisabeth; Mencken, Thomas; Atkins, Charity; Liu, Qi; Rabindran, Sridhar; Kumar, Rakesh; Hong, Xuan; Goetz, Aaron; Stanley, Thomas; Taylor, J. David; Sigethy, Scott D.; Tomberlin, Ginger H.; Hassell, Annie M.; Kahler, Kirsten M.; Shewchuk, Lisa M.; Gampe, Robert T. Journal of Medicinal Chemistry, 2012 , vol. 55, # 16 p. 7193 - 7207 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |