54739-18-3
| Name | fluvoxamine |
|---|---|
| Synonyms |
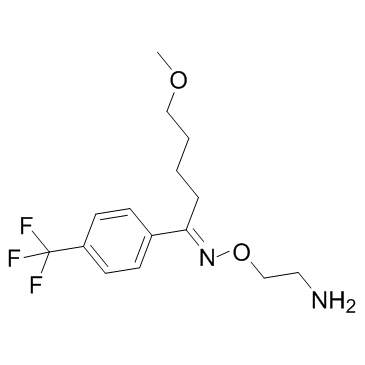
2-{[(E)-{5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino]oxy}ethanamine
(E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]-1-pentanone O-(2-Aminoethyl)oxime Faverin Floxyfral 1-Pentanone, 5-methoxy-1-[4-(trifluoromethyl)phenyl]-, O-(2-aminoethyl)oxime, (1E)- Dumirox Maveral 5-methoxy-1-[4-(trifluoromethyl)-phenyl]-1-pentanone-0-(2-aminoethyl)oxime 2-[({(1E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino)oxy]ethanamine Luvox 1-Pentanone, 5-methoxy-1-(4-(trifluoromethyl)phenyl)-, O-(2-aminoethyl)oxime, (E)- MFCD00865345 5-methoxy-1-[4-(trifluoromethyl)-phenyl]-1-pentanone O-(2-aminoethyl)oxime Fevarin 2-[[5-methoxy-1-[4-(trifluoromethyl)phenyl]-pentylidene]amino]oxyethanamine (1E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentanal O-(2-aminoethyl)oxime 5-Methoxy-4'-(trifluoromethyl)valerophenone (E)-O-(2-aminoethyl)oxime Fluvoxamine (E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]-1-pentanoneo-(2-aminoethyl)oxime |
| Description | Fluvoxamine is an antidepressant which functions pharmacologically as a selective serotonin reuptake inhibitor.Target: SSRIsFluvoxamine is effective in inhibiting 5-ht uptake by blood platelets and brain synaptosomes. The antagonism by fluvoxamine of the reserpine-induced lowering of the pentamethylenetetrazole convulsive threshold can be regarded as due to an effect upon 5-HT uptake. In contrast to the effects of desmethylimipramine and imipramine, no stimulatory effects are found in rats when rapidly acting reserpine-like compounds are given following a dose of fluvoxamine [1]. fluvoxamine appears to improve combat-related PTSD symptoms but not depressive symptoms. The high attrition rate and lack of a placebo group limits the conclusions of our study. Controlled studies of fluvoxamine in the treatment of PTSD are warranted [2]. Fluvoxamine was less potent at decreasing ethanol self-administration when food was available concurrently versus when ethanol was available in isolation [ED50: 4.0 (2.7-5.9) and 5.1 (4.3-6.0)]. Effects on food were similar under each condition in which food was available. The results demonstrate that the potency of fluvoxamine in reducing ethanol-maintained behavior depends on whether ethanol is available in isolation or in the context of concurrently scheduled food reinforcement [3].Clinical indications: Depression; Obsessive compulsive disorder; Social phobia FDA Approved Date: December 5, 1994Toxicity: Anorexia, Constipation, Dry mouth, Headache, Nausea, Nervousness, Skin rash, Sleep problems, Somnolence, Liver toxicity, Mania, Increase urination, Seizures, Sweating increase, Tremors, or Tourette's syndrome. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 370.6±52.0 °C at 760 mmHg |
| Melting Point | 120-122.5ºC |
| Molecular Formula | C15H21F3N2O2 |
| Molecular Weight | 318.335 |
| Flash Point | 177.9±30.7 °C |
| Exact Mass | 318.155518 |
| PSA | 56.84000 |
| LogP | 3.11 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.474 |
| Storage condition | -20°C Freezer, Under Inert Atmosphere |
| HS Code | 2928000090 |
|---|
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |