1391-36-2
| Name | Duramycin |
|---|---|
| Synonyms |
UNII-BPR0F3X56H
Lancovutide [INN] Ancovenin,2-L-lysine-6-(3-aminoalanine)-10-L-phenylalanine-12-L-phenylalanine-13-L-valine-15-(erythro-3-hydroxy-L-aspartic acid)-,cyclic 6-19)-imine Leucopeptin LANCOVUTIDE |
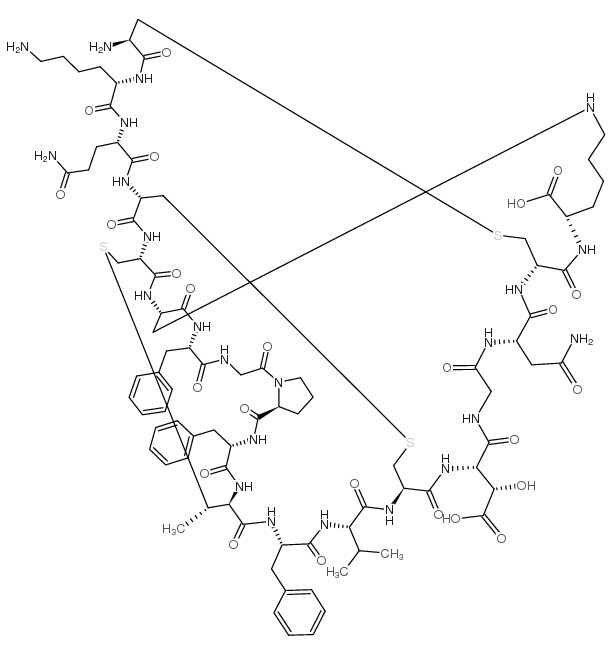
| Description | Duramycin (Moli1901;Lancovutide) is a cyclic peptide lantibiotic derived from Streptomyces cinnamoneuma. Duramycin stimulates chloride secretion in airway epithelium and has the potential for cystic fibrosis treatment. Duramycin interacts with phosphatidylethanolamine (PE) and has antibacterial, antiviral effects[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Duramycin does stimulate Cl- efflux from cystic fibrosis bronchial epithelial cells (CFBE) in a narrow concentration range (around 1 μM). However, 100 and 250 μM of Duramycin inhibits Cl- efflux from CFBE cells. The [Ca2+]i is increased by 3 μM Duramycin in 16HBE cells, but decreases after 1, and 3 μM of Duramycin in CFBE cells[1]. Duramycin affects protein-lipid interactions, impairs protein translocation, induces aggregation of lipid vesicles, and inhibits ATP-dependent Ca2+ uptake by the sarcoplasmic reticulum[1]. |
| In Vivo | Evaluation of [68Ga]NODAGA-Duramycin as a positron emission tomography (PET) tracer of cell death for whole-body detection of chemotherapy-induced organ toxicity. Organ uptake is analyzed in untreated and doxorubicin, busulfan, and cisplatin-treated mice 2 h after intravenous injection of [68Ga]NODAGA-Duramycin. [68Ga]NODAGA-Duramycin PET/CT is successfully applied to non-invasively detect chemotherapy-induced organ toxicity with high sensitivity in mice[3]. |
| References |
| Density | 1.0238 (rough estimate) |
|---|---|
| Melting Point | 271-273ºC |
| Molecular Formula | C89H125N23O25S3 |
| Molecular Weight | 1999.25000 |
| Exact Mass | 1997.82000 |
| PSA | 835.99000 |
| Index of Refraction | 1.6680 (estimate) |
| Storage condition | 2-8°C |
| Water Solubility | 0.1 M HCl: soluble10mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | BV7100000 |