125-13-3
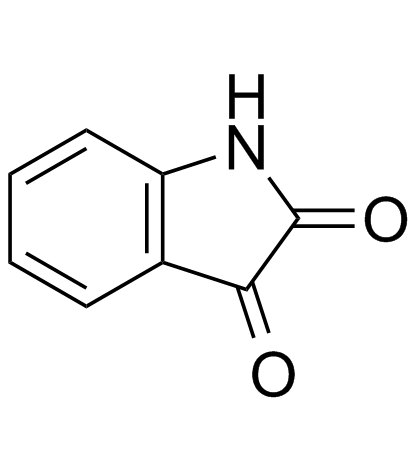
| Name | 3,3-bis(4-hydroxyphenyl)-1H-indol-2-one |
|---|---|
| Synonyms |
Phenolisatin
Oxyphenisatine Propellax Hoscolax Neodrast 3,3-bis-(4-hydroxy-phenyl)-indolin-2-one EINECS 204-728-1 Veripaque 3,3-bis(4'-hydroxyphenyl)-2-oxo-2,3-dihydroindole Diphenolisatin 3,3-bis-(4-hydroxy-phenyl)-1,3-dihydro-indol-2-one Normalax Oxyphenisatin Isatinbisphenol 3,3-bis-(4-hydroxyphenyl)-2-indolinone |
| Description | Oxyphenisatine (Oxyphenisatin) is a laxative. Oxyphenisatin acetate is the pro-drug of oxyphenisatin with anticancer activity. |
|---|---|
| Related Catalog | |
| In Vitro | Oxyphenisatin has been shown to have antiproliferative activity. Oxyphenisatin acetate (OXY, NSC 59687) is the pro-drug of oxyphenisatin. OXY inhibits the growth of the breast cancer cell lines MCF7, T47D, HS578T, and MDA-MB-468 (IC50=0.8, 0.6, 2.1, 1.8 μM). This effect is associated with selective inhibition of translation accompanied by rapid phosphorylation of the nutrient sensing eIF2α kinases, GCN2 and PERK[1]. |
| In Vivo | Toxicity studies demonstrate that mice tolerate IP administration of OXY at 300 mg/kg once daily or 200 mg/kg twice daily. Administration of OXY at 300 mg/kg IP once daily for 10 days results in significantly smaller tumors from day 33 to day 52[1]. |
| Animal Admin | Mice: When tumors reaches 120 mg, mice are randomized into treatment groups and therapy is initiated. Asimple toxicity assessment to determine tolerability to OXY is conducted by administering single intraperitoneal (IP) doses of compound at 100, 200, and 400 mg/kg. The mice were observed for adverse effects for 14 days postdose[1]. |
| References |
| Density | 1.352g/cm3 |
|---|---|
| Boiling Point | 562.9ºC at 760 mmHg |
| Molecular Formula | C20H15NO3 |
| Molecular Weight | 317.33800 |
| Flash Point | 294.2ºC |
| Exact Mass | 317.10500 |
| PSA | 69.56000 |
| LogP | 3.52230 |
| Vapour Pressure | 2.82E-13mmHg at 25°C |
| Index of Refraction | 1.691 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933790090 |
|---|
|
~90% 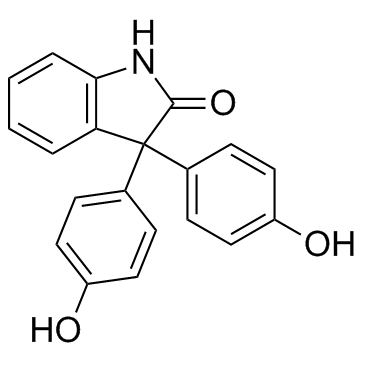
125-13-3 |
| Literature: Song, Hyun Nam; Lee, Hong Jung; Kim, Hyoung Rae; Ryu, Eung K.; Kim, Jae Nyoung Synthetic Communications, 1999 , vol. 29, # 19 p. 3303 - 3311 |
|
~% 
125-13-3 |
| Literature: Woelm Fabr. Chem.-Pharm. Praep. Patent: DE824203 , 1948 ; |
|
~% 
125-13-3 |
| Literature: Inagaki Yakugaku Zasshi, 1933 , vol. 53, p. 686,696;dtsch.Ref.S.133 Chem. Zentralbl., 1933 , vol. 104, # II p. 2133 |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |