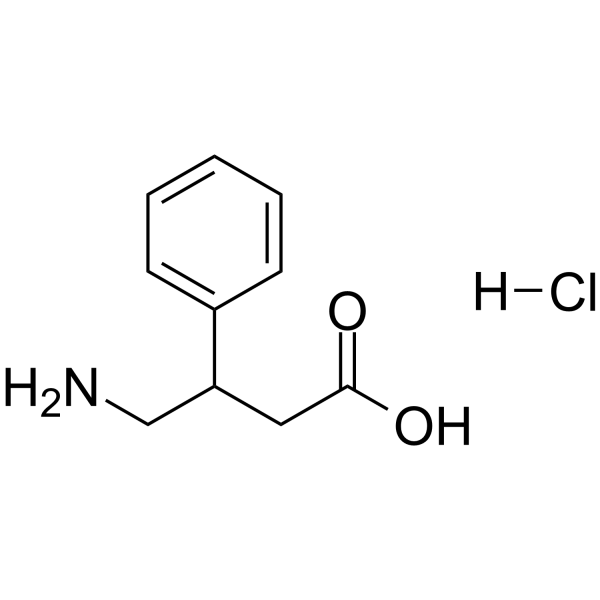
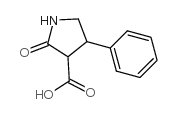
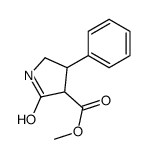
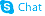77472-70-9
| Name | 2-(2-Oxo-4-phenylpyrrolidin-1-yl)acetamide |
|---|---|
| Synonyms |
fonturacetam
UNII:99QW5JU66Y Carphedon Phenylpiracetam 4-Phenyl-2-pyrrolidone-1-acetamide 2-(2-Oxo-4-phenyl-1-pyrrolidinyl)acetamide 1-Pyrrolidineacetamide, 2-oxo-4-phenyl- 2-(2-oxo-4-phenylpyrrolidin-1-yl)acetamide 4-Phenylpiracetam Phenotropil |
| Description | Phenylpiracetam(Phenotropyl; Phenotropil) is a phenylated derivative of the nootropic drug piracetam. It is used as a stimulant nootropic drug that can be up to 30-60 times more potent than piracetam.IC50 Value:Target: AMPA receptor allosteric modulatorin vitro: N/Ain vivo: In the open-field test, a significant increase in locomotor activity was observed after a single administration of R-phenotropil at doses of 10 and 50 mg/kg and S-phenotropil at a dose of 50 mg/kg. In the forced swim test, R-phenotropil induced an antidepressant effect at doses of 100 and 50 mg/kg, and S-phenotropil was active at a dose of 100 mg/kg. R-phenotropil significantly enhanced memory function in a passive avoidance response test at a dose of 1 mg/kg; the S-enantiomer did not show any activity in this test [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 486.4±45.0 °C at 760 mmHg |
| Molecular Formula | C12H14N2O2 |
| Molecular Weight | 218.252 |
| Flash Point | 247.9±28.7 °C |
| Exact Mass | 218.105530 |
| PSA | 64.39000 |
| LogP | 0.16 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.580 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|
|
~99% 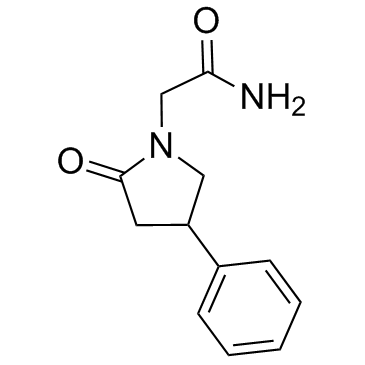
77472-70-9 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 14, # 11 p. 776 - 780 Khimiko-Farmatsevticheskii Zhurnal, , vol. 14, # 11 p. 43 - 48 |
|
~81% 
77472-70-9 |
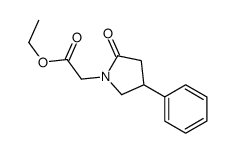
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 58, # 5 p. 1093 - 1102,970 - 979 |
|
~% 
77472-70-9 |
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 58, # 5 p. 1093 - 1102,970 - 979 |
|
~% 
77472-70-9 |
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 58, # 5 p. 1093 - 1102,970 - 979 |
|
~% 
77472-70-9 |
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 58, # 5 p. 1093 - 1102,970 - 979 |
|
~% 
77472-70-9 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 14, # 11 p. 776 - 780 Khimiko-Farmatsevticheskii Zhurnal, , vol. 14, # 11 p. 43 - 48 |
|
~% 
77472-70-9 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 14, # 11 p. 776 - 780 Khimiko-Farmatsevticheskii Zhurnal, , vol. 14, # 11 p. 43 - 48 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |