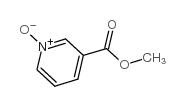
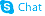1986-81-8
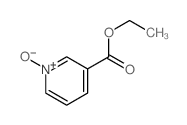
| Name | 1-oxidopyridin-1-ium-3-carboxamide |
|---|---|
| Synonyms |
3-carbamoylpyridine 1-oxide
Nicotinamide,1-oxide nicotinamide N1-oxide EINECS 217-859-4 3-Pyridinecarboxamide,1-oxide 1-Oxy-nicotinamide Nicotinic acid amide N-oxide 3-Pyridincarboxamid-1-oxid Nicotinamide N-oxide 3-Pyridinecarboxamide,Oxynicotinamide MFCD00006202 |
| Description | Nicotinamide N-oxide, an in vivo nicotinamide metabolite, is a potent, and selective antagonist of the CXCR2 receptor. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Nicotinamide is one of the forms of vitamin B3. It is a precursor for nicotinamide adenine dinucleotide, which is best known as an electron carrier in oxidative phosphorylation and as a cofactor for many dehydrogenases. It is metabolized through two enzymatic systems. The first system starts with the methylation of nicotinamide by nicotinamide N-methyltransferase, which can subsequently be oxidized by aldehyde oxidase. The second enzymatic system oxidizes nicotinamide to nicotinamide N-oxide[1]. A series of nicotinamide N-oxides is synthesized and shown to be novel, potent, and selective antagonists of the CXCR2 receptor. Compound 1 has demonstrated potent inhibition of neutrophil chemotaxis (IC50=10 nM). Compound 2 is a selective antagonist of IL-8 binding (IC50=110 nM) and potent inhibitor of neutrophil chemotaxis (IC50=170 nM)[2]. |
| References |
| Density | 1.34 g/cm3 |
|---|---|
| Boiling Point | 514.7ºC at 760 mmHg |
| Melting Point | 291-293 °C (dec.)(lit.) |
| Molecular Formula | C6H6N2O2 |
| Molecular Weight | 138.12400 |
| Flash Point | 265.1ºC |
| Exact Mass | 138.04300 |
| PSA | 68.55000 |
| LogP | 0.91430 |
| Vapour Pressure | 1.05E-10mmHg at 25°C |
| Index of Refraction | 1.602 |
| Storage condition | Refrigerator |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~95% 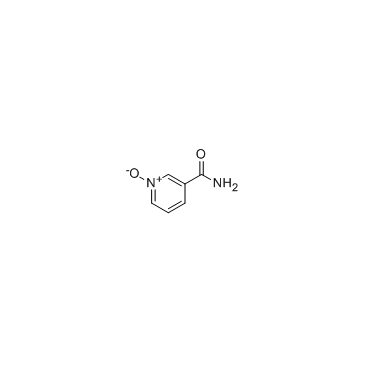
1986-81-8 |
| Literature: Rout, Laxmidhar; Punniyamurthy, Tharmalingam Advanced Synthesis and Catalysis, 2005 , vol. 347, # 15 p. 1958 - 1960 |
|
~55% 
1986-81-8 |
| Literature: Moehrle, Hans; Niessen, Robert Zeitschrift fur Naturforschung - Section B Journal of Chemical Sciences, 2000 , vol. 55, # 5 p. 434 - 442 |
|
~% 
1986-81-8 |
| Literature: Dissertationes Pharmaceuticae, , vol. 9, p. 197,203 Chem.Abstr., , p. 6337 |
|
~% 
1986-81-8 |
| Literature: Yakugaku Zasshi, , vol. 72, p. 1474,1476 Chem.Abstr., , p. 8077 |
|
~% 
1986-81-8 |
| Literature: Yakugaku Zasshi, , vol. 72, p. 1474,1476 Chem.Abstr., , p. 8077 |