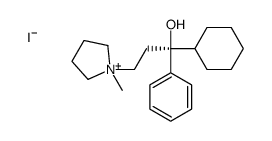
77-37-2
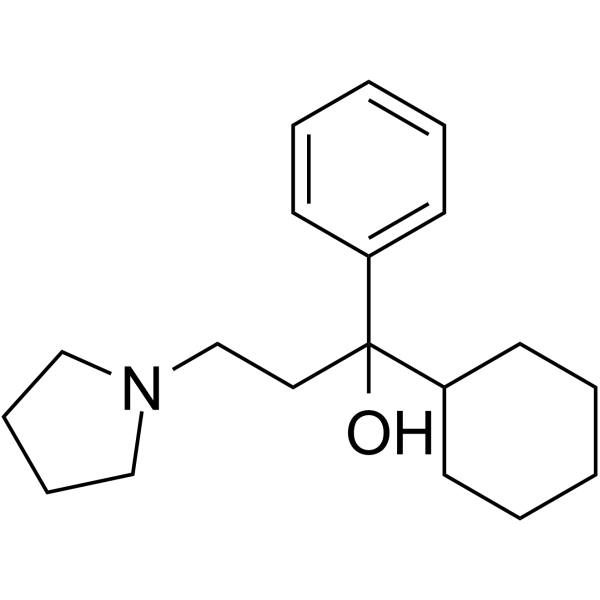
| Name | procyclidine |
|---|---|
| Synonyms |
Kemadrine
Arpicolin Procyklidin Spamol 1-cyclohexyl-1-phenyl-3-pyrrolidin-1-yl-propan-1-ol 1-Cyclohexyl-3-pyrrolidino-1-phenyl-propan-1-ol Lergine Elorine 1-cyclohexyl-1-phenyl-3-pyrrolidin-1-ylpropan-1-ol Triciclidina tricyclamol Osnervan 1-Cyclohexyl-1-phenyl-3-pyrrolidino-propan-1-ol |
| Description | Procyclidine (Tricyclamol; (±)-Procyclidine), an anticholinergic agent, is a muscarinic receptor antagonist that also has the properties of an N-methyl-D-aspartate (NMDA) antagonist. Procyclidine can be used in studies of Parkinson's disease and related psychiatric disorders such as Soman-induced epilepsy[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Procyclidine (subcutaneous injection, 0.3-6.0 mg/kg) in combination with physostigmine (PhS) increases protection in a dose-dependent manner in rats and guinea pigs infected with soman and can prevent seizures altogether[1]. Animal Model: Male Sprague-Dawley rats, Dunkin-Hartley male guinea pigs[1] Dosage: 0.3-6.0 mg/kg Administration: Subcutaneous injection; once Result: Increased protection, resulting in 1.92, 2.24, 3.95 and 5.07 fold in rats, 3.00, 3.25, 4.50 and 4.70 fold in guinea pigs at the doses of 0.3, 1.0, 3.0 or 6.0 mg/kg, respectively. Protected the neurological integrity of the brain and prevented Soman-induced severe brain damage in the hippocampus, cortex, amygdala and thalamus. |
| References |
| Density | 1.057 g/cm3 |
|---|---|
| Boiling Point | 433.5ºC at 760 mmHg |
| Melting Point | 85.5-86.5° |
| Molecular Formula | C19H29NO |
| Molecular Weight | 287.44000 |
| Flash Point | 205.7ºC |
| Exact Mass | 287.22500 |
| PSA | 23.47000 |
| LogP | 3.87830 |
| Index of Refraction | 1.554 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
77-37-2 |
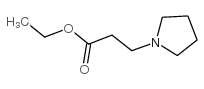
| Literature: Adamson et al. Journal of the Chemical Society, 1951 , p. 52,58 |
|
~% 
77-37-2 |
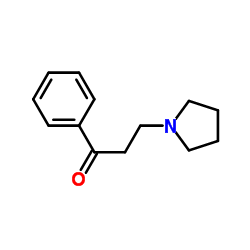
| Literature: Ratouis; Combes Bulletin de la Societe Chimique de France, 1959 , p. 576,578 |
|
~% 
77-37-2 |
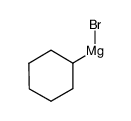
| Literature: Adamson et al. Journal of the Chemical Society, 1951 , p. 52,58 |
| Precursor 4 | |
|---|---|
| DownStream 2 | |