Alcohol oxidase
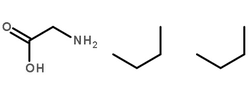
Alcohol oxidase structure
|
Common Name | Alcohol oxidase | ||
|---|---|---|---|---|
| CAS Number | 9073-63-6 | Molecular Weight | 191.311 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C2H5NO2.2[C4H10] | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of Alcohol oxidaseAlcohol oxidase is a functional enzyme of methanol utilization pathway and can be isolated from yeast peroxisome[1]. |
| Name | Oxidase, Alcohol |
|---|---|
| Synonym | More Synonyms |
| Description | Alcohol oxidase is a functional enzyme of methanol utilization pathway and can be isolated from yeast peroxisome[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C2H5NO2.2[C4H10] |
|---|---|
| Molecular Weight | 191.311 |
| InChIKey | LJCNDNBULVLKSG-UHFFFAOYSA-N |
| SMILES | CCCC.CCCC.NCC(=O)O |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
[Effect of nitrogen source on gene expression of first steps of methanol utilization pathway in Pichia pastoris].
Genetika 49(4) , 454-60, (2013) Pichia pastoris is a methylotrophic yeast that is widely used for the expression of heterologous proteins. Here, we have investigated the dependence of AOX1 and CAT1 expression on the source of nitrog... |
|
|
Heterologous protein expression in Pichia thermomethanolica BCC16875, a thermotolerant methylotrophic yeast and characterization of N-linked glycosylation in secreted protein.
FEMS Microbiol. Lett. 334(2) , 127-34, (2012) This study describes Pichia thermomethanolica BCC16875, a new methylotrophic yeast host for heterologous expression. Both methanol-inducible alcohol oxidase (AOX1) and constitutive glyceraldehyde-3-ph... |
|
|
Improvement of porcine interferon-α production by recombinant Pichia pastoris via induction at low methanol concentration and low temperature.
Appl. Biochem. Biotechnol. 165(2) , 559-71, (2011) Improved porcine interferon-α (pIFN-α) production by recombinant Pichia pastoris was achieved by culture conditions optimization in a 5-l bioreactor. The results indicated that the pIFN-α concentratio... |
| MFCD00130452 |

