O-Acetylvanillin
Modify Date: 2025-08-27 13:14:15
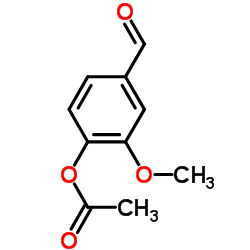
O-Acetylvanillin structure
|
Common Name | O-Acetylvanillin | ||
|---|---|---|---|---|
| CAS Number | 881-68-5 | Molecular Weight | 194.184 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 288.5±25.0 °C at 760 mmHg | |
| Molecular Formula | C10H10O4 | Melting Point | 77-79 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 124.9±23.2 °C | |
Use of O-AcetylvanillinVanillin acetate is easily synthesized from vanillin by treatment with acetic anhydride[1]. |
| Name | vanillin acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Vanillin acetate is easily synthesized from vanillin by treatment with acetic anhydride[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 288.5±25.0 °C at 760 mmHg |
| Melting Point | 77-79 °C(lit.) |
| Molecular Formula | C10H10O4 |
| Molecular Weight | 194.184 |
| Flash Point | 124.9±23.2 °C |
| Exact Mass | 194.057907 |
| PSA | 52.60000 |
| LogP | 1.10 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.540 |
| InChIKey | PZSJOBKRSVRODF-UHFFFAOYSA-N |
| SMILES | COc1cc(C=O)ccc1OC(C)=O |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2915390090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2915390090 |
|---|---|
| Summary | 2915390090. esters of acetic acid. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:5.5%. General tariff:30.0% |
|
Azlactones and phenylacetic acids derived from the 2-nitro-derivatives of vanillin, isovanillin, and veratraldehyde.
J. Chem. Soc. 174 , 376-8, (1948)
|
|
|
The discovery-oriented approach to organic chemistry. 6. Selective reduction in organic chemistry: Reduction of aldehydes in the presence of esters using sodium borohydride. Baru AR and Mohan RS
J. Chem. Educ. 82(11) , 1674, (2005)
|
|
|
New synthesis of 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone and hexahydrocoenzyme Q4 chromanol. Weinstock LM, et al.
J. Chem. Eng. Data 12(1) , 154-55, (1967)
|
| EINECS 212-920-1 |
| 4-Acetoxy-3-Methoxybenzaldehyde |
| Vanillin, acetate |
| Vanillin, acetate (8CI) |
| 3-methoxy-4-acetyloxybenzaldehyde |
| 4-ACETYLVANILLIN |
| acetic acid 4-formyl-2-methoxy-phenyl ester |
| 3-Methoxy-4-acetoxybenzaldehyde |
| MFCD00003362 |
| 4-Formyl-2-methoxyphenol acetate |
| Benzaldehyde, 4-(acetyloxy)-3-methoxy- |
| Acetovanillin |
| 4-(Acetyloxy)-3-methoxybenzaldehyde |
| acetyl vanillin |
| 4-Formyl-2-methoxyphenyl acetate |
| 4-O-Acetylvanillin |
| Benzaldehyde, 4- (acetyloxy)-3-methoxy- |
| O-Acetylvanillin |
| Vanillin Acetate |
| FEMA 3108 |
 CAS#:121-33-5
CAS#:121-33-5 CAS#:75-36-5
CAS#:75-36-5 CAS#:108-24-7
CAS#:108-24-7![4-[tert-butyl(dimethyl)silyl]oxy-3-methoxybenzaldehyde Structure](https://image.chemsrc.com/caspic/266/69404-94-0.png) CAS#:69404-94-0
CAS#:69404-94-0![Methanediol,1-[4-(acetyloxy)-3-methoxyphenyl]-, 1,1-diacetate Structure](https://image.chemsrc.com/caspic/207/6301-73-1.png) CAS#:6301-73-1
CAS#:6301-73-1 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:54145-18-5
CAS#:54145-18-5 CAS#:7368-78-7
CAS#:7368-78-7 CAS#:90-05-1
CAS#:90-05-1 CAS#:52783-83-2
CAS#:52783-83-2 CAS#:60632-40-8
CAS#:60632-40-8 CAS#:2973-75-3
CAS#:2973-75-3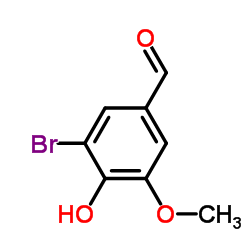 CAS#:2973-76-4
CAS#:2973-76-4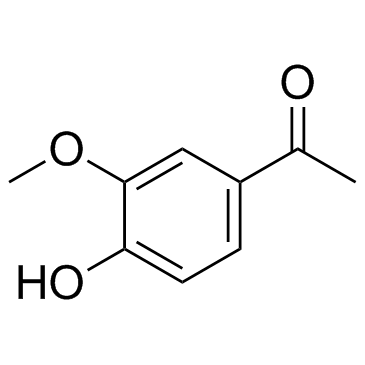 CAS#:498-02-2
CAS#:498-02-2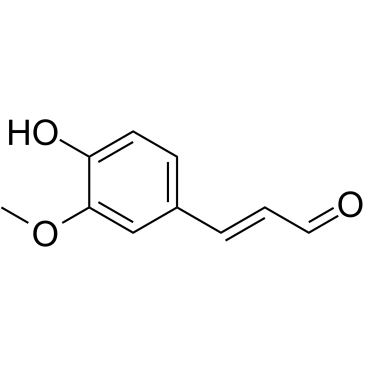 CAS#:458-36-6
CAS#:458-36-6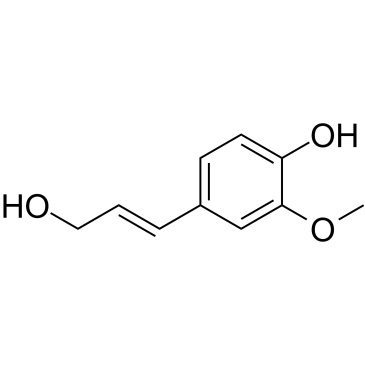 CAS#:458-35-5
CAS#:458-35-5 CAS#:18692-68-7
CAS#:18692-68-7 CAS#:2432-60-2
CAS#:2432-60-2
