Acetytastragaloside
Modify Date: 2025-08-22 18:19:32
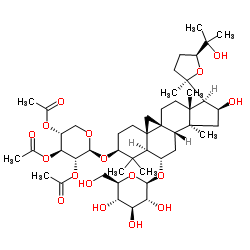
Acetytastragaloside structure
|
Common Name | Acetytastragaloside | ||
|---|---|---|---|---|
| CAS Number | 84687-47-8 | Molecular Weight | 911.080 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 902.9±65.0 °C at 760 mmHg | |
| Molecular Formula | C47H74O17 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 254.5±27.8 °C | |
Use of AcetytastragalosideAcetylastragaloside I is a glycoside that can be isolated from the roots of Astragalus baibutensis. Acetylastragaloside I is the first cycloartane-type triterpene with remarkable trypanocidal activity with IC50 values of 9.5 and 5.0 μg/mL for T. brucei rhodesiense and T. cruzi, respectively. Acetylastragaloside I can be used for the research of trypanosome infection[1]. |
| Name | acetylastragaloside I |
|---|---|
| Synonym | More Synonyms |
| Description | Acetylastragaloside I is a glycoside that can be isolated from the roots of Astragalus baibutensis. Acetylastragaloside I is the first cycloartane-type triterpene with remarkable trypanocidal activity with IC50 values of 9.5 and 5.0 μg/mL for T. brucei rhodesiense and T. cruzi, respectively. Acetylastragaloside I can be used for the research of trypanosome infection[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 9.5 μg/mL (T. brucei rhodesiense), 5.0 μg/mL (T. cruzi), >30 μg/mL (L. donovani), >20 μg/mL (P. falciparum), 24.2 μg/mL (L6 cells)[1] |
| In Vitro | Acetylastragaloside I (0.123-90 μg/mL; 72-96 h) shows in vitro antiprotozoal activity to T. brucei rhodesiense, T. cruzi, L. donovani and P. falciparum with IC50 values of 9.5, 5.0, >30 and >20 μg/mL, respectively[1]. Acetylastragaloside I (0.123-90 μg/mL; 72 h) exhibits cytotoxicity effects to rat skeletal myoblasts (L6) cells[1]. Cell Cytotoxicity Assay[1] Cell Line: Rat skeletal myoblasts (L6) cell line Concentration: 0.123-90 μg/mL Incubation Time: 72 hours Result: Showed cytotoxicity to L6 cells with an IC50 value of 24.2 μg/mL. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 902.9±65.0 °C at 760 mmHg |
| Molecular Formula | C47H74O17 |
| Molecular Weight | 911.080 |
| Flash Point | 254.5±27.8 °C |
| Exact Mass | 910.492615 |
| PSA | 246.43000 |
| LogP | 3.10 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.586 |
| InChIKey | KWZSMZJAHIHRRT-UHFFFAOYSA-N |
| SMILES | CC(=O)OC1COC(OC2CCC34CC35CCC3(C)C(C6(C)CCC(C(C)(C)O)O6)C(O)CC3(C)C5CC(OC3OC(CO)C(O)C(O)C3O)C4C2(C)C)C(OC(C)=O)C1OC(C)=O |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| β-D-Glucopyranoside, (3β,6α,9β,16β,20R,24S)-20,24-epoxy-16,25-dihydroxy-3-[(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)oxy]-9,19-cyclolanostan-6-yl |
| Acetytastragaloside |
| (3β,6α,9β,16β,20R,24S)-16,25-Dihydroxy-3-[(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)oxy]-20,24-epoxy-9,19-cyclolanostan-6-yl β-D-glucopyranoside |
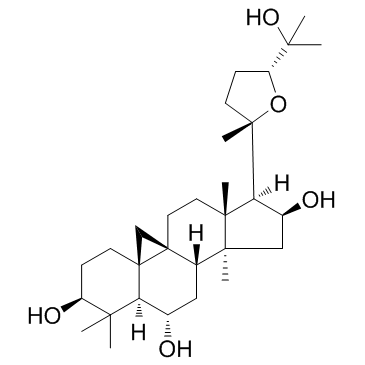 CAS#:84605-18-5
CAS#:84605-18-5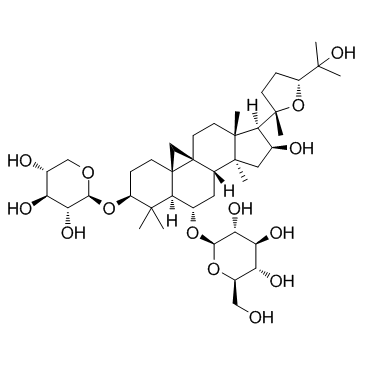 CAS#:83207-58-3
CAS#:83207-58-3
