17-AAG
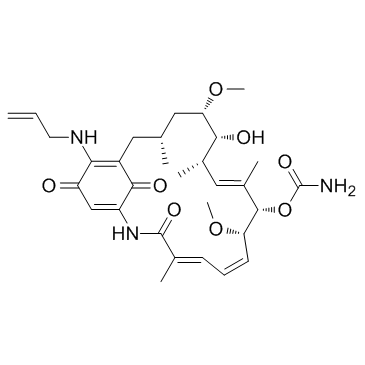
17-AAG structure
|
Common Name | 17-AAG | ||
|---|---|---|---|---|
| CAS Number | 75747-14-7 | Molecular Weight | 585.688 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 797.8±60.0 °C at 760 mmHg | |
| Molecular Formula | C31H43N3O8 | Melting Point | 201-203ºC | |
| MSDS | USA | Flash Point | 436.3±32.9 °C | |
Use of 17-AAG17-AAG is a potent HSP90 inhibitor with an IC50 of 5 nM, having a 100-fold higher binding affinity for tumour cell derived HSP90 than normal cell derived HSP90. |
| Name | tanespimycin |
|---|---|
| Synonym | More Synonyms |
| Description | 17-AAG is a potent HSP90 inhibitor with an IC50 of 5 nM, having a 100-fold higher binding affinity for tumour cell derived HSP90 than normal cell derived HSP90. |
|---|---|
| Related Catalog | |
| Target |
HSP90:5 nM (IC50) Mitophagy Autophagy |
| In Vitro | 17-AAG causes the degradation of HER2, Akt, and both mutant and wild-type AR and the retinoblastoma-dependent G1 growth arrest of prostate cancer cells. 17-AAG inhibits prostate cancer cell lines with IC50s ranged from 25-45 nM (LNCaP, 25 nM; LAPC-4, 40 nM; DU-145, 45 nM; and PC-3, 25 nM)[1]. Combination of 17-AAG (10 nM) and Trastuzumab induces more effective ErbB2-degradation. 17-AAG (0.1-1 μM) induces a nearly complete loss of ErbB2 on ErbB2-overexpressing breast cancer cells[2]. 17-AAG inhibits cell growth and induces G2/M cell cycle arrest and apoptosis in CCA cells together with the down-regulation of Bcl-2, Survivin and Cyclin B1, and the up-regulation of cleaved PARP[3]. |
| In Vivo | 17-AAG (25-200 mg/kg, i.p.) causes a dose-dependent decline in AR, HER2, and Akt expression in prostate cancer xenografts. 17-AAG treatment at doses sufficient to induce AR, HER2, and Akt degradation results in the dose-dependent inhibition of androgen-dependent and -independent prostate cancer xenograft growth without toxicity[1]. 17-AAG (60 mg/kg) with paclitaxel (60 mg/kg) and rapamycin (30 mg/kg) inhibits A549 and MDA-MB-231 tumor growth far more potently than paclitaxel-containing micelles and effected tumor cures in MDA-MB-231 tumor-bearing animals by tail vein injection[4]. |
| Cell Assay | For the Alamar Blue proliferation assay, 2-4×103 cells are plated in 96-well plates. Later (48 h), cells are treated with 17-AAG for 96 h or 0.01% DMSO as control. On day 4, Alamar Blue viability assay is performed as described elsewhere. IC50 and IC90s are calculated as the doses of 17-AAG required to inhibit cell growth by 50 and 90%, respectively. Cell cycle distribution is assayed as described previously with a Becton Dickinson fluorescence-activated cell sorter and analyzed by the Cell Cycle Multicycle system. |
| Animal Admin | 17-AAG is dissolved in an EPL vehicle. To aid in the identification of an optimal dose and schedule, nontumor bearing mice are treated by i.p. injection with 25-200 mg/kg of 17-AAG 5 days/week for 3 weeks or by the EPL vehicle alone. Serum samples are taken from each group, and equal volumes are pooled on days 5, 10, and 15 of treatment for serum chemistry and liver function analysis. At sacrifice, plasma samples are collected for complete blood count. A gross necropsy is performed on all of the mice, and a complete necropsy, including histopathology, is performed on 1 animal/group. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 797.8±60.0 °C at 760 mmHg |
| Melting Point | 201-203ºC |
| Molecular Formula | C31H43N3O8 |
| Molecular Weight | 585.688 |
| Flash Point | 436.3±32.9 °C |
| Exact Mass | 585.304993 |
| PSA | 166.28000 |
| LogP | 2.68 |
| Vapour Pressure | 0.0±6.4 mmHg at 25°C |
| Index of Refraction | 1.566 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 22-24/25-36-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29419090 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |
|
Interactome analysis of the human respiratory syncytial virus RNA polymerase complex identifies protein chaperones as important cofactors that promote L-protein stability and RNA synthesis.
J. Virol. 89(2) , 917-30, (2015) The human respiratory syncytial virus (HRSV) core viral RNA polymerase comprises the large polymerase protein (L) and its cofactor, the phosphoprotein (P), which associate with the viral ribonucleopro... |
|
|
HGUE-C-1 is an atypical and novel colon carcinoma cell line.
BMC Cancer 15 , 240, (2015) Colorectal carcinoma is a common cause of cancer. Adjuvant treatments include: 5-fluorouracil administered together with folinic acid, or more recently, oral fluoropyrimidines such as capecitabine, in... |
|
|
Comparative Study of 17-AAG and NVP-AUY922 in Pancreatic and Colorectal Cancer Cells: Are There Common Determinants of Sensitivity?
Transl. Oncol. 7(5) , 590-604, (2014) The use of heat shock protein 90 (Hsp90) inhibitors is an attractive antineoplastic therapy. We wanted to compare the effects of the benzoquinone 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanesp... |
| 17-AAG,17AAG |
| 17-AG |
| 2-azabicyclo[16.3.1]docosa-2,4,6,10,18,21-hexaene-20,22-dione, 3,13-dihydroxy-9-(hydroxyiminomethoxy)-8,14-dimethoxy-4,10,12,16-tetramethyl-19-(2-propen-1-ylamino)-, (2E,4E,6Z,8S,9S,10E,12S,13R,14S,16R)- |
| 17-AGG |
| 17-AAG (Tanespimycin) |
| KOS 953 |
| (4E,6Z,8S,9S,10E,12S,13R,14S,16R)-19-(Allylamino)-13-hydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate |
| MFCD04973892 |
| 2-Azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione, 9-[(aminocarbonyl)oxy]-13-hydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-19-(2-propen-1-ylamino)-, (4E,6Z,8S,9S,10E,12S,13R,14S,16R)- |
| Telatinib |
| (4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-19-(prop-2-en-1-ylamino)-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate |
| [3H]-17-AAG |
| 17-demethoxygeldanamycin |
| 17-allylamino-17-demethoxygeldanamycin |
| Tanespimycin |
| 17-AAG |
| Gld-36 |
| 17-AAG (KOS953) |
 CAS#:107-11-9
CAS#:107-11-9 CAS#:30562-34-6
CAS#:30562-34-6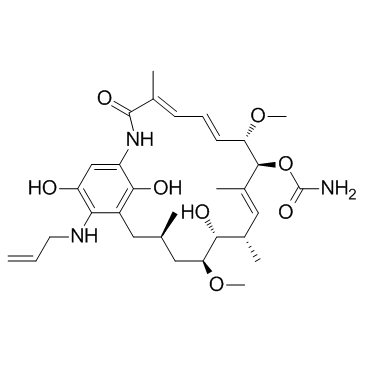 CAS#:857402-23-4
CAS#:857402-23-4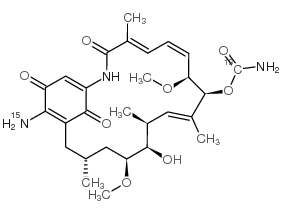 CAS#:64202-81-9
CAS#:64202-81-9
